II.4.3 In vitro dissolution
4.3.1 Methods
· Preparation of dissolution
medium
98.64 ml of 37% hydrochloric acid was diluted to 10.0L with
distilled water. The resulting 0.1N hydrochloric acid solution was used as
dissolution medium.
· Calibration curve
Stock solution
40 mg of metronidazole was accurately weighed, dissolved in
dissolution medium and sonicated for about 5 min to give a 25 ml solution
having a concentration of 1600 mg/l.
5 ml from this solution was diluted to 50.0 ml with
dissolution medium to give a stock solution with a concentration of 160
mg/l.
Standard solutions
0.5, 0.75, 1, 2 and 3 ml of the stock solution were separately
diluted with dissolution medium to 10.0 ml. The standard solutions obtained had
concentrations of 8, 12, 16, 32 and 48 mg/l, respectively.
A calibration curve (absorbance vs. concentration) y =
0.0355x + 0.0114 with a correlation coefficient (R2) of 0.9998 was
constructed.
· Dissolution testing
Dissolution profiles were determined using the USP basket
method (Method 1). Each of 6 tablets was added to a basket connected to a
stirring shaft which was placed inside a dissolution vessel filled with 900ml
of dissolution medium maintained at 370.5°C. The rotation speed of the
basket was 100 rpm. At 10, 20, 30, 40, 50 and 60 minutes, 5ml samples were
withdrawn, filtered, diluted 20 times and analysed spectrophotometrically at
278nm.
4.2.2 Results
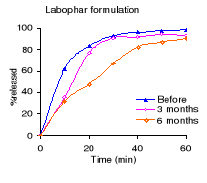
Table 4.2 shows the percentage dissolved within 60 minutes of
dissolution testing and Figure 4.1 the different dissolution profiles. Before
stability testing all drugs complied with the USP 24 dissolution requirements
(not less than 80% of the labelled amount should dissolve within 60 minutes).
The amount of drug released after 60 minutes of dissolution test was more than
90% for all formulations. The Holden Medica formulation did not withstand the
storage at high temperature and high relative humidity: the percentage released
being outside the specifications after 6 months.
Table 4.2: Percentage of metronidazole dissolved within 60
minutes of dissolution testing before and after 3 and 6 months of storage at
40°C and 75% RH. USP requirements: more than 80 % released within 60
minutes.
Manufacturer % of the
labelled amount released
0 months
3 months 6 months
Elys chemicals (Elogyl) 97.8
99.8 88.7
Labophar 98.2
92.6 90.1
Holden medica 95.3
87.8 66.9


Figure 4.1: Dissolution profiles of metronidazole formulations
before and after 3 and 6 months of storage at 40°C and 75 % RH.
| 


