II.2.3 In vitro dissolution
2.3.1 Methods
· Preparation of dissolution
medium
The dissolution medium consisted of 0.05M acetate buffer
prepared as follows: 9 g of anhydrous sodium acetate was dissolved in 800 ml
distilled water, 8.3 ml of glacial acetic acid was added. The resulting
solution was diluted to 5.0L.
· Calibration curve
Stock solution
35 mg of acetylsalicylic acid reference powder was accurately
weighed and transferred to a 100.0 ml volumetric flask. 1 ml of methanol was
added, then about 50 ml of dissolution medium. The mixture was sonicated for
about 2 min. The solution was diluted to 100.0 ml using the dissolution medium
to obtain a stock solution with a concentration of 350 mg of acetylsalicylic
acid / l.
Standard solutions
3, 4, 5, 7 and 9 ml were separately diluted to 10.0 ml using
the dissolution medium; the resulting standard solutions had concentrations of
105, 140, 175, 245 and 315 mg/l acetylsalicylic acid, respectively. Absorbances
of those solutions were spectrophotometrically measured at 265nm. A calibration
curve (absorbance vs. acetylsalicylic acid concentration) y = 0.0027x + 0.0031
with a correlation coefficient (R2) of 0.9998 was constructed.
Dissolution testing
Dissolution profiles were determined using the USP basket
method (Method 1). Each of 6 tablets was added to a basket fixed to a stirring
shaft, placed inside a dissolution vessel (filled with 900 ml of dissolution
medium maintained at 37°C 0.5°C) and rotated at a speed of 50 rpm. At
different time intervals (5, 10, 15, 20, 25 and 30 min) 5ml filtered samples
were manually withdrawn, diluted twice with dissolution medium and
spectrophotometrically analysed at 265 nm. Concentrations were calculated from
the above mentioned calibration curve.
2.3.2 Results
The dissolution profiles for each formulation before and after
3 and 6 months of accelerated stability testing are shown in Figure 2.1 and the
percent drug released after 30 minutes in Table 2.3. Before stability testing
the S&R formulation did not disintegrate, while others complied with the
USP 24 requirements (not less than 80% dissolved within 30 minutes). After six
months of stability testing, only the Bayer formulation remained compliant with
the USP 24 requirements. The percentage released for Minasprin formulation
decreased, however it remained compliant with the USP 24 requirements. The
release rate of the B.J International formulation decreased dramatically.
Table 2.3 Percentage of acetylsalicylic acid dissolved within
30 minutes of dissolution testing before and after 3 and 6 months of storage at
40°C and 75% RH. USP requirements: more than 80 % released within 30
minutes.
Manufacturer % of the
labelled amount per tablet
0 months
3 months 6 months
Bayer 99.0
97.2 95.6
BJ international 84.7
71.8 34.3
Girlloh (Minasprin) 97.2
80.5 76.5
S&R (Saraprin) 5.1
- -
Not analyzed for 3 and 6 months.
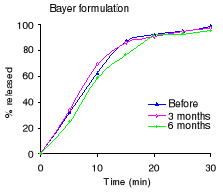


Figure 2.1 Dissolution profiles of acetylsalicylic acid before
and after 3 and 6 months of storage at 40°C and 75 % RH:
| 


