|
REPUBLIC OF CAMEROON REPUBLIQUE DU CAMEROUN
Peace - Work - Fatherland Paix - Travail -
Patrie
FACULTY OF MEDICINE AND BIOMEDICAL SCIENCES
THE
UNIVERSITY OF YAOUNDE I
DEPARTMENT OF PAEDIATRICS
POST-GRADUATE
COURSE
ACADEMIC YEAR 1996-1997
POSTERIOR URETHRAL VALVES IN
CHILDREN:
A review of 28 cases in Yaounde,
Cameroon
Thesis Submitted in Partial Fulfilment for
the
Requirements of End of Course Diploma
(Specialist Diploma in Clinical
Sciences, Option Paediatrics)
By
Dr. CHIABI Andreas TEHJI
Paediatric
Resident
DIRECTOR CO-DIRECTORS
Prof. ZOUNG KANYI Jimmy Dr. FRU ANGWAFO III
Dr. Prof. ABENA OBAMA Marie
Thérèse
1
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
TABLE OF CONTENTS
PAGE
DEDICATIONS 2
ACKNOWLEDGEMENTS 3
LIST OF PERSONNEL FMBS . .5
LIST OF ABBREVLATIONS 10
RESUME .. 12
SUMMARY . .17
CHAPTER I : I NTRODUCTION .. 21
CHAPTER II : OBJECTIVES 24
REVIEW OF LITERATURE 26
CHAPTER III :
Anatomy of the normal urethra 27
Embryology of the urinary system .27
Embryogenesis of posterior urethral valves 35
Classification of posterior urethral valves .35
Patho-physiological changes induced by posterior
urethral
valves on the urogenital tract .. ..38
III -- A
|
:
|
|
III -- B
|
:
|
|
III -- C
|
:
|
|
III -- D
|
:
|
|
III -- E
|
:
|
|
CHAPTER IV
|
:
|
|
CHAPTER V
|
:
|
|
CHAPTER VI
|
:
|
|
CHAPTER V1I
|
:
|
|
CHAPTER VIII
|
:
|
|
CHAPTER IX
|
:
|
M ATERIALS AND METHODS 42
R ESULTS .45
D ISCUSSION .66
C ONCLUSIONS AND RECOMMENDATIONS ......78
B IBLIOGRAPHY 81
APPENDIX 91
2
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
DEDICATIONS



To The ALMIGHTY GOD:
I pray you continue to help and guide me in my daily and
professional activities so that the future be brighter and life more
worthwhile.
To my Family:
My wife - Ema, my sons - Edmond and Roland.
Thanks for the perseverance and may we hope for brighter days in the
nearest future.
To my mum - NDISI Tabitha, late Dad CHIABI David,
Uncle NGONG Mathias and all my brothers and sisters. This work is
entirely the fruit of your sacrifice
To all children with posterior urethral valves.
I love you and I promise to take special care of you
3
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
ACKNOWLEDGEMENTS







To Dr ANGWAFO III
Thanks for encouraging me to do this work despite all the
difficulties I had. You have made me learn and understand a bit of Paediatric
Urology. Thanks for everything.
To Dr ABENA OBAMA Marie Thérèse
Thanks for accepting to supervise this work. I highly
appreciate the advice and encouragement you gave me. All through my residency
you had been like a mother to me.
To Prof ZOUNG KANYI Jimmy
Thanks for accepting to supervise this work despite your tight
schedule. Thanks very much indeed.
Thanks to all my teachers who moulded me up into a Paediatrician,
especially
Prof TETANYE EKOE, Dr ABENA OBAMA, Dr DOUMBE Pierre,
Dr KAGO Innocent, Dr MBONDA Elie, Dr TCHOKOTEU Pierre Fernand, Dr TIETCHE
Felix, Dr MONEBENIMP Francisca, Dr ONDOA MEKONGO Martin, Dr YAP John, Dr
NSANGOU Innoussa. I hope to apply all the technical skills you taught
me in examining and treating my patients.
Special thanks to Dr TCHOKOTEU Pierre Fernand
and Dr ENGOUDOU née Douala MOUTENG Valentine of the
General Hospital. My first staggering steps in Paediatrics were with you when I
was still premature. You encouraged me to go on.
Thanks to Dr KAMDEM Annie and husband
Mr Jean Paul KAMDEM, Dr Amos KAMDEM. Thanks
for all the material and moral support you gave me in the most difficult
moments of my life. I pray our friendship blossom as the morning roses.
Thanks to my friends Mr EYÀA Jean Dominique, Mr
KAMTO Victor, Mr KEUKAM Justin, Mr TCHOUGA Phillipe, Mr NANKAM Bernard, Mr
KEMAJOU Augustin. You were close to me in very trying moments of my
career. Accept my sincere thanks
4
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|



Thanks to my long time friends Dr TAKOU Virgine, Dr
BIBEE MAYI, Dr TSIAGADIGUI Jean Gustave, Dr MBANGTANG
Celestine (University of Zimbabwe), Dr NYOM Elizabeth
(FMBS). It has been very rough but worth the while.
Thanks to Mr and Mrs Leo ANGUO for the material
and moral support.
Finally, sincere thanks to Miss Evelyn BANINLA
and Mr MBUYONGHA Nico for sparing their time to type and
arrange this work.
5
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
LISTE DU PERSONNEL
ADMINISTRATIF ET
ENSEIGNANT
I PERSONNEL ADMINISTRATIF
1 SOSSO Maurice Doyen.
2 NGU BLACKETT Kathleen Vice-Doyen Chargée des
Affaires
Académiques et de la Coopération.
3 BENGONO née CISSE TOURE Vice-Doyen
Chargée de la Scolarité et
des Statistiques.
4 NDUMBE Peter Vice-Doyen Chargée de la
Recherche.
S EWOLO NOMO DAF
6 MBARGA BEKONO Chef de Service Financier
7 ABENA Marie- Thérèse Chef de Service de
Stage
S DONGMO Louis Chef de Service de Programmes
9 BOUMSONG Vincent Bibliothécaire en Chef
II PERSONNEL ENSEIGNANT a)
PROFESSEURS
1 ABONDO Antoine Anatomie Pathologique
2 EDZOA Titus Chirurgie Générale
3 EIMO MALONGA Elisée Chirurgie Générale
4 HAGBE Paul Médecine Interne / Cardiologie
5 KAPTUE NOCHE Lazare Hématologie
6 LANTUM NONI Daniel Santé Publique
7 MAKANG MA MBOG Mathias Neuropsychiatrie
S MBEDE Joseph Pédiatrie
9 NGU BLACKETT Kathleen Médecine Interne / Cardiologie
10 NGU LIFANJI Jacob Médecine Interne
/Néphrologie
11 NKOULOU Hubert Pédiatrie
12 OBOUNOU AKONG Dominique Anatomie Humaine
13 ZOUNG KANYI Jimmy Chirurgie/Urologie
6
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
|
b)
|
MAITRES DE CONFERENCES
|
|
|
1
|
ASONGANYI TAZOACHA
|
Biochimie / Immunologie
|
|
2
|
ATCHOU Guillaume
|
Physiologie Humaine
|
|
3
|
BEJANGA Beltus
|
Chirurgie Généra/e
|
|
4
|
BENGONO née CISSE TOURE Geneviève
|
O.R.L.
|
|
5
|
DJOUMESSI Sosthène
|
Biochimie
|
|
6
|
DOH Anderson SAMA
|
Gynécologie /Obstétrique
|
|
7
|
DONGMO Louis
|
Anatomie / Neurologie
|
|
8
|
GONSU FOTSIN Joseph
|
Radiologie/Imagerie Médicale
|
|
9
|
JATO Johnson GAMNGONG
|
Chimie Pharmaceutique
|
|
10
|
JUIMO Alain Georges
|
Radiologie /Imagerie Médicale
|
|
11
|
KAMDOM MOYO Joseph
|
Gynécologie /Obstétrique
|
|
12
|
KOUEKE Paul
|
Dermatologie / Vénérologie
|
|
13
|
LEKE Robert Ivo
|
Gynécologie /Obstétrique
|
|
14
|
MBAKOP André
|
Anatomie Pathologique
|
|
15
|
MUNA Walinjom
|
Médicine Interne / Cardiologie
|
|
16
|
NKAM Maurice
|
Pharmacologie /Thérapeutique
|
|
17
|
NDJITOYAP NDAM Elie - Claude
|
Médecine Interne /Gastro - entérologie
|
|
18
|
NDUMBE Peter
|
Microbiologie / Immunologie
|
|
19
|
NGOGANG Jeanne
|
Biochimie
|
|
20
|
NGUIMBOUS Jean François
|
Chirurgie Thoracique / Cardio vasculaire
|
|
21
|
NJIKAM KAYA Lawrence
|
Pharmacie Galénique
|
|
22
|
SAME - EBOKO Albert
|
Parasitologie
|
|
23
|
SOSSO Maurice
|
Chirurgie Générale
|
|
24
|
TETANYE EKOE
|
Pédiatrie
|
|
25
|
TSALA MBALA Pierre
|
Physiologie Humaine
|
|
26
|
YOUMBISSI TCHETAGNI Joseph
|
Médecine Interne / Néphrologie
|
c) CHARGES DE COURS
1 ABENA OBAMA Marie-Thérèse
Pédiatrie
2 ABOLO MBENTI Louis Chirurgie Générale
3 AFANE ELA Anatole Anesthésie -
Réanimation
4 AFANE ZE Emmanuel Médecine Interne /
Pneumologie
5 ANGWAFO III FRU Chirurgie / Urologie
7
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
|
5 BINAM née NGO NJOM Fidèle
7 BIOUELE MEVÀA Jean Moïse
8 BIWOLE SIDA Magloire
9 DIFFANG Charles
|
Anesthésie- Réanimation Anesthésie-
Réanimation
Médecine Interne / Gastro-entérologie
Médecine Légale
|
|
|
10
|
DOUMBE Pierre
|
Pédiatrie
|
|
|
11
|
ESSAME OYONO Jean Louis
|
Anatomie Pathologique
|
|
|
12
|
ETAME EWANE
|
Sociologie Médicale
|
|
|
13
|
FOGAM Eric GALABE
|
Gynécologie -Obstétrique
|
|
|
14
|
FOMULU Joseph Nelson
|
Gynécologie -Obstétrique
|
|
|
15
|
FOUDA ONANA Alexandre
|
O.R.L.
|
|
|
16
|
JATO Miriam NGWANG
|
Education pour la Santé'
|
|
|
17
|
KAGO Innocent
|
Pédiatrie
|
|
|
18
|
KOUAM Luc
|
Gynécologie -Obstétrique
|
|
|
19
|
KOUDA ZEH Alexandre
|
Médecine Interne / Gastro-entérologie
|
|
|
20
|
KOULLA née SHIRO Sinata
|
Microbiologie
|
|
|
21
|
KUABAN Christopher
|
Médecine Interne /
Pneumol. et Med. du
Trav.
|
|
|
22
|
LANDO Gabriel
|
Biochimie / Immunologie
|
|
|
23
|
LEKE née GANA FOMBAN Rose
|
Parasitologie / Immunologie
|
|
|
24
|
LOHOUE née PETMY Julienne
|
Parasitologie / Mycologie
|
|
|
25
|
MASSO MISSE Pierre
|
Chirurgie Générale
|
|
|
26
|
MBAKOP Gabriel
|
Physiologie
|
|
|
27
|
MBANYA Jean Claude
|
Médecine interne /Endocrinologie
|
|
|
28
|
MBONDA Elie
|
Neuro-Pédiatrie
|
|
|
29
|
MELI Jean
|
Santé Publique
|
|
|
30
|
MOUAMPEA MBIO Marie Claire
|
Anatomie Pathologie
|
|
|
31
|
MOUKOURI Ernest
|
Ophtalmologie
|
|
|
32
|
MOYOU SOMO Roger
|
Parasitologie
|
|
|
33
|
NDOBO Pierre
|
Médecine Interne / Cardiologie
|
|
|
34
|
NDOUMOU Alain
|
Médecine Interne / Pneumologie
|
|
|
35
|
NGASSA CHANCHU Pius
|
Gynécologie -Obstétrique
|
|
|
36
|
NKO'O AMVENE Samuel
|
Radiologie / Imagerie Médicale
|
|
|
37
|
OYONO ENGUELE Samuel
|
Physiologie Humaine
|
|
|
38
|
POLL GOUATER Henri
|
Biochimie
|
|
|
39
|
SIMO MOYO Justin
|
Anesthésie / Réanimation
|
|
|
40
|
SOW MAMADOU
|
Chirurgie / Uro1ogie
|
|
|
41
|
TAGNY ZUKAM David
|
Radiologie / Imagerie Médicale
|
|
|
42
|
TAKONOMO Samuel
|
Chirurgie Générale
|
|
|
43
|
TAKOR TAKOR Samuel
|
Histologie / Embryologie
|
|
|
44
|
TAPKO Jean-Baptiste
|
Hématologie / Immunologie
|
|
|
45
|
TCHOKOTEU Pierre Fernand
|
Pédiatrie
|
|
|
46
|
TEYANG Abel
|
Chirurgie Thoracique et
|
|
|
|
Cardio-vasculaire
|
|
|
47
|
TIETCHE Félix
|
Pédiatrie
|
|
|
|
|
8
|
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
48 WAMBA TEMGOUA Maurice
Gynécologie-Obstétrique
49 YOMI Jean Radiologie / Radiothérapie
d) ASSISTANTS
|
1
|
ADIOGO Dieudonné
|
Microbiologie
|
|
2
|
AMANA Jean Paul
|
Radiologie/Imagerie Diagnostique
|
|
3
|
ANYANGWE née NWIGWE Stella
|
Santé Publique
|
|
4
|
BELLEY PRISO Eugène
|
Gynécologie-Obstétrique
|
|
5
|
BEFIDI MENGUE née NJEE N B, Rosa
|
Parasitologie
|
|
6
|
BIYIHA Dieudonné
|
Anesthésie-Réanimation
|
|
7
|
BOB' OYONO Jean Marie
|
Anatomie/Chirurgie Pédiatrique
|
|
8
|
DONG à ZOK
|
Biophysique /Médecine Nucléaire
|
|
9
|
EBANA
|
Ophtalmologie
|
|
10
|
ELOUNDOU
|
Neuro-chirurgie
|
|
11
|
ESSOMBA Arthur
|
Chirurgie Générale
|
|
12
|
ETOM EMPIME
|
Neuro-chirurgie
|
|
13
|
KASIA Jean-Marie
|
Gynécologie-Obstétrique
|
|
14
|
KINGUE Samuel
|
Médecine Interne / Cardiologie
|
|
5
|
LOLO Berthe
|
Psychiatrie
|
|
16
|
MBANYA née SHU Dora
|
Hématologie
|
|
17
|
MBU Robinson ENOW
|
Gynécologie-Obstétrique
|
|
18
|
MELAMAN SEGO Frédéric
|
Physiologie
|
|
19
|
MONEBENIMP Franscisca
|
Pédiatrie
|
|
20
|
MONNY LOBE Marcel
|
Hématologie
|
|
21
|
MOUELLE SONE
|
Radiothérapie
|
|
22
|
MOUSSALA
|
Ophtalmologie
|
|
23
|
NJEE BUGHA Théodore
|
Neuro-chirurgie
|
|
24
|
NOUEDOUI Christophe
|
Médecine Interne / Endocrinologie
|
|
25
|
NJOYA OUDOU
|
Médecine Interne / Gastro -entérologie
|
|
26
|
NSANGOU INNOUSSA
|
Pédiatrie
|
|
27
|
NTONE ENYIME Félicien
|
Psychiatrie
|
|
28
|
ONDOA MEKONGO Martin
|
Pédiatrie
|
|
29
|
ONDOBO ANDZE Gervais
|
Chirurgie Pédiatrique
|
|
30
|
SENDE Charlotte
|
Radiologie/Imagerie Médicale
|
|
31
|
SHASHA VIBAN Willibroad
|
Gynécologie-Obstétrique
|
|
32
|
TCHOUNWOU Paul Bernard
|
Environnement /Toxicologie
|
|
33
|
WANKAH Christian
|
Santé Publique
|
9
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
e) CYCLE DES ETUDES SUPERIEURES EN SOINS
INFIRMIERES
(CESSI)
1 MBONDA Elie
2 BOLANGA Elise (Mme)
3 NGUEMATCHA Julienne
4 ASSOMOU MBA Lydienne
5 NOUMSI André
6 OUSMANOU NASSOURO
7 OMOLOKO Cécile
8 KAMTA Charles
10
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
ABBREVIATIONS
BUN Blood Urea Nitrogen
CBC Complete Blood Count
DMSA Dimercapto -- Succinic Acid
FG Filtration Glomerulaire
GFR Glomerular Filtration Rate
IVP Intra Venous Pyelography
IVSD Intra-Ventricular Septal Defect
K+ Potassium
NCHS National Centre for Health Statistics
PUV Posterior Urethral Valves
RBC Red Blood Count
TUR Trans-Urethral Resection
UPJ Uretero - Pelvic Junction
UTI Urinary Tract Infection
UVI Urographie Intraveineuse
V Vesicostomy
VCUG Voiding Cystourethrogram
VUR Vesico - Ureteral Reflux
WBC White Blood Count
11
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
«Posterior urethral valves is a heterogeneous
disorder with a sequelae ranging from voiding dysfunction without renal
impairment to early onset of renal failure and death»
DENES et al 1997 (1)
« the picture as usually described is but one
end of a spectrum and there are
many less severe and dramatic cases which escape
recognition»
HENDREN 1971 (2)
12
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|

RESUME
13
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
Nous avons étudié 28 cas d'enfants
traités ou suivis pour valves de l'urètre postérieure du
ler Janvier 1985 au 31 Décembre 1996 au CHU, à
l'Hôpital Central et à l'Hôpital Général de
Yaoundé.'
Nos objectifs ont été d'étudier les
aspects épidémiologiques des valves de l'urètre
postérieure à Yaoundé, de décrire la
présentation c1inique, les procédures diagnostiques et le devenir
post-chirurgical en termes de fonction rénale, de croissance et
d'anornalies urinaires chez ces enfants
L'étude a comporté 2 phases: une
rétrospective transversale et l'autre prospective longitudinale
descriptive, durant lesquelles nous avons étudié les
données cliniques (anamnèse, procédures diagnostiques,
traitement et suivi). Ce suivi comportait la surveillance clinique du jet
urinaire, du poids, de la taille, des complications post-opératoires, de
l'urée et la créatinine sanguine, ainsi que de l'uroculture.
L'âge des patients à la première
consultation après le début des symptômes variait entre 1
jour et 8 ans (moyenne 1,6 ans). L'age des patients au moment du diagnostic
variait entre 9 jours et 13 ans (moyenne 2.9 ans). L'intervalle moyen entre
l'âge à la première consultation et l'âge au moment
du diagnostic était de 9.7 mois.
Le diagnostic des valves a été fait par
échographie chez 3 patients (sur la base de l'hydronéphrose
bilatérale, vessie de lutte et dilatation de l'urètre
postérieure). Chez les 25 patients restants le diagnostic a
été fait à la fois par l'échographie et par la
cystographie mictionnelle
Considérant l'âge des patients au moment du
diagnostic, ceux-ci ont été' divisés en trois groupes :
Groupe I : (âge inférieur à 1 mois au moment
du diagnostic): 5 patients. Groupe II (âge compris entre 1 mois et 12
mois): 9 patients.
Groupe III: (âge supérieur à 12 mois au
moment du diagnostic): 14 patients. Dans les antécédents, on note
le plus souvent des infections urinaires à répétition
(50%), une hypertension artérielle chez 7% des patients (en insuffisance
rénale terminale).
Les symptômes urinaires les plus fréquemment
retrouvés sont la miction « goutte-à-goutte » (60.7%),
dysurie (54%) et rétention urinaire (25%). Les symptômes
14
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
extra-urinaires les plus fréquents sont la fièvre
(25%) et 'un retard de croissance (25%).
Les principaux signes physiques sont: hernies ombilicales (21%)
et distensions vésicales (10.7%). Une ascite urinaire est
retrouvée chez 2 patients.
Nous avons pu avoir les résultats d'uroculture chez 19
de nos patients: 12 étaient stériles; chez les 7 autres, les
germes retrouvés étaient des bactéries
Gram-négatif: E. coli (26%), Pseudomonas aeroginosa
(11 %), Moraxella (11%), Klebsiella pneumoniae (11 %),
Enterobacter aerogenes (5 %) et Proteus mirabilis (5%).
La fonction rénale au moment du diagnostic a
été appréciée par le calcul de la filtration
glomérulaire (F.G) (à partir de la formule de COCKCROFT) Elle
était très altérée avec une F.G à 5ml/min/
1.73m2 dans le Groupe I, à 14ml/min/1.73m2 dans le
Groupe II et 19m1/min/l.73m2 dans le Groupe III.
Cependant, 12 patients seulement ont été revus pour
évaluation dans la phase prospective et parmi eux, 9 patients seulement
ont fait les tests de fonction rénale.
Nous avons comparé la F.G au moment du diagnostic et a
l'évaluation finale chez ces 9 patients.
On a noté une amélioration de la fonction
rénale chez 6 patients (66,7%) avec une augmentation de la F.G. moyenne
passant de 23.7m1/min/1 73m2 à 58,8ml/min/1.73m2.
Chez 2 patients (22%), on a noté une détérioration de a
fonction rénale avec une F.G moyenne passant de
53,5m1/min/1,73m2 à 33ml/min/1.73m2. Chez 2
patients, la F.G est restée stable à
15ml/min/1,73m2
Une analyse comparative du poids (au moment du diagnostic et
de l'évaluation finale) a également été faite chez
9 patients. Ces poids ont été reportés sur les courbes de
croissance de la NCHS (National Center for Health Statistics). Au moment du
diagnostic, 8 de ces 9 patients avaient un retard de croissance
inférieur au 50ème percentile. A l'évaluation finale nous
avons noté une amélioration de La croissance chez 5 patients mais
seulement 2 sont passés au dessus de 50ème percentile.
L'urographie intraveineuse (UIV) a été faite
chez 6 patients et a montré une uretèro -hydronéphrose
bilatérale chez 5 patients (83%), un retard de sécrétion
chez 1 patient (17%) et un rein gauche muet chez 1 patient 17%.
La scintigraphie a été faite chez 2 patients et
chez l'un d'eux, il y avait une forte suspicion de dysplasie rénale
15
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
L'exploration urodynamique de la vessie a été faite
chez 2 patients et a montré une réduction de la compliance
vésicale chez l'un des patients
En ce qui concerne le traitement, 26 patients ont subi une
intervention chirurgicale les 2 patients restants ayant été
perdus de vue après le diagnostic.
Vingt patients ont eu une ablation endoscopique des valves, 4 une
vesicostomie de Blocksom, 3 une cystostomie et 2 une ablation par sonde.
Les interventions chirurgicale secondaires ont
été: urétéroplastie (3), nephrostomie (4),
circoncisions (4), urétérostomie (4), diverticulectomie (5) et
urétérostomie pour sténose urétrale secondaire a
une ablation par sonde (6).
Lors de l'évaluation finale, nous avons noté 6
décès (21%), 10 perdus de vue (36%) et 12 revus à la phase
prospective de l'étude. Les causes de décès ont
été : septicémie 3 cas (50%), syndrome de levée
d'obstacle, 2 cas (33%) et insuffisance rénale chronique, 1 cas
(17%).
A la fin de l'étude, nous arrivons à la
conclusion que les valves de l'urètre postérieure sont
diagnostiquées tardivement au Cameroun, quand l'insuffisance
rénale et le retard de croissance sont déjà
avancés. Le suivi des ces patients est insuffisant principalement parce
que cette pathologie aussi bien que ses répercussions sur la fonction
rénale et la croissance ne sont pas bien comprises.
Ainsi nous recommandons que




Le jet urinaire des enfants soit évalué
cliniquement lors de consultations
Toute infection urinaire chez l'enfant soit correctement
investiguée (surtout à l'échographie) car elle peut
être la première manifestation des valves de l'urètre
postérieur ou d'une autre uropathie obstructive.
Les complications des valves de l'urètre postérieur
et leur prise en charge soient bien connues.
Un effort soit fait par les obstétriciens, les
pédiatres et les radiologues afin qu'un diagnostic précoce,
puisse être posé pour qu'une prise en charge adéquate soit
instituée dans les plus brefs délais.
16
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|

SUMMARY
17
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
We reviewed the files of 28 children treated or followed up
for posterior urethral valves (PUV) from 1st January 1985 to the 31st of
December 1996 in the University Teaching Hospital, Central Hospital and the
General Hospital in Yaoundé.
Our specific objectives were to review the epidemiological
aspects of PUV in Yaoundé, assess the clinical presentation, diagnostic
procedures and outcome following surgery in terms of renal function, patient
growth and urinary abnormalities.
The study was a retrospective cross-sectional and a
prospective longitudinal descriptive review of clinical data, during which the
history diagnostic procedures, treatment and follow-up parameters were noted;
(stream, weight, height, BUN, creatinine, urine cultures and post - operative
complications).
The mean age of the patients at diagnosis was 2.9 years (range
9 days to 13 years) and the mean age at first consultation after onset of
symptoms was 1.6 years (range 1 day to 8 years). The mean interval between age
of first consultation and age at diagnosis was 9.7 months.
The diagnosis of PUV was made on ultrasound in 3 patients on
the basis of bilateral uretero - hydronephrosis, thick - wall trabeculated
bladder and a dilated posterior urethra. In the remaining 25, diagnosis was
made on both ultrasound and voiding cystourethrograms. Considering the age of
diagnosis, the patients were divided into three groups: Group I (age of
diagnosis less than 1 month) 5 patients; Group II (1 month -12 months) 9
patients and Group III (age greater than 12 months) 14 patients.
The past history showed mostly recurrent urinary tract
infection (UTI) in 50% of the patients and hypertension in 7% of the patients
who had end-stage renal failure. The most frequent urinary symptoms were
dribbling (60.7%), dysuria (54%) and urine retention (25%) whereas the most
frequent non-urinary symptoms were fever (25%) and failure to thrive (25%). The
main physical findings were umbilical hernias (21%) and bladder distension
(10.7%), urinary ascitis was present in 2 patients (7%).
Results of urine cultures were available in 9 patients, 12
were sterile Pathogens cultured in 7 patients were gram negative bacteria: E.
coli (26%) Pseudomonas aeroginosa (11%), Moraxella (11%),
Kiebsiella pneumoniae (11%), Enterobacter aerogenes (5%), and
Proteus mirabilis (5%).
18
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
Renal function at diagnosis , assessed from the
Glomerular-Filtration Rate (GFR) (calculated from COCKCROFT'S FORMULA ) was
markedly impaired with a GFR at 5 ml/min/1.73m2 in Group I,
14m1/min/1.73m2 in Group II and 19 ml/min/1.73m2 in Group
III. However, 12 patients turned up for evaluation in the prospective phase of
the study and only 9 could do renal function tests. We compared the GFR at
diagnosis and at final evaluation in these 9 patients. 6 (66.7%) had improved
renal function with a mean GFR increasing from 23.7 ml/min/l.73m2 to
58.8 ml/min/1.75m2. 2 (22%) had deteriorated renal function with a
mean GFR dropping from 53.5 ml/min to 33 rnl/min/l.73m2 whereas in 2
the GFR remained stable, at 15m1/min/l.73m2.
Comparative weight analysis (at diagnosis and at final
follow-up) was also done in the same 9 patients. The weights were plotted onto
NCHS (National Centre for Health Statistics) growth charts. At diagnosis 8 of
the 9 patients (88.9%) had growth retardation with weights below the
50th percentile. At final evaluation 5 patients had improved growth,
but only 2 had gone above the 50th percentile.
Intravenous pyelography (IVP) was done in 6 patients and it
showed bilateral uretero-hydronephrosis in 5 (83%), late secretion in 1(17%)
and a non-functioning left kidney in 1(17%). Scintigraphy was done in 2
patients and in 1 there was a strong suspicion of dysplastic kidneys. Bladder
urodynamic studies were undertaken in 2 patients and reduced bladder compliance
was noted in 1.
Concerning treatment 26 patients underwent surgery. 2 were
lost to follow up after diagnosis. 20 patients underwent endoscopic valve
ablations, 4 Blocksom vesicostomies, 3 cystostomies and 2 catheter ablations.
Secondary procedures performed were: ureterosplasty (3), nephrostomy (4),
circumcisions (4), ureterostomy (4), diverticulectomy (5) and urethrostomy [for
meatal stenosis following catheter ablations] (6).
At final evaluation we noted 6 deaths (21%). 10 lost to
follow-up (36%) and 12 reassessed. Causes of the deaths were septicemia: 3
cases (50%), post-obstructive diuresis: 2 cases (33 %) and chronic renal
failure: 1 case (17%). 8 cases of incontinence were noted in the whole
series.
At the end of the study we arrived at the conclusions that PUV
in Cameroon are
19
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
still diagnosed very late with renal impairment and growth
retardation already advanced. Follow-up of these patients is inadequate mainly
because the pathology is not well understood as well as its repercussions on
renal function and growth.
We thus recommend that:



The urinary stream of children be clinically evaluated in routine
consultations.
Any urinary tract infection in a child be adequately
investigated (especially with ultrasound) as it might be the first
manifestation of PUV or any other obstructive uropathy.
The complications of PUV and their management be well known
An effort be made by obstetricians, paediatricians and
radiologists in making early diagnosis so that appropriate management be
started as soon as possible.
20
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|

INTRODUCTION
21
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
Posterior Urethral Valves (PUV) are congenital membranous
recesses in the posterior urethra in males (7). They are the most common cause
of lower urinary tract obstruction in male infants (7,8,9,10,11,12,
13,14,15,16,19).
The incidence of PUV is reported to be 1 in 8000 live births
according to CASALE A.J. cited in (10) and 1 in 25 000 live births according to
ATWEL J.D. (4). The incidence in Oman is reported to be 1 in 2375 new-born
males (16) which is considerably higher than any previously reported series.
This was associated with an increased rate of consanguinity but there was no
clear pattern of inheritance. In Yaoundé, they constitute the second
cause of obstructive uropathies (1 3.3%) after uretero-pelvic junction
obstruction (14.6%) according to NNOMZO'O (62) whereas it represents 15.22% of
all uropathies in children in Côte d'Ivoire (18).
In infants especially neonates, the clinical presentation may
be atypical in the form of diarrhoea, vomiting, fever, convulsions, abdominal
masses (hydronephrotic kidneys, distended bladder, foetal or neonatal urinary
ascitis with retroperitoneal urinomas), failure to thrive or even sepsis (11,
13, 19, 20, 22). Some new-borns who present an unexpected respiratory distress
syndrome and/or an unexplained pneumothorax or pneumomediastinum, may be found
to have obstructive uropathy - usually posterior urethral valves and pulmonary
hypoplasia (8, 11). In older boys, the presenting symptoms are usually
recurrent urinary tract infections and dribbling or straining to urinate (8,
11, 20, 21, 22). In small infants, the condition may be so advanced when first
seen, that renal failure secondary to gross bilateral hydroureters and
hydronephrosis dominates the clinical picture (22). Clinical suspicion may be
missed especially in neonates because of the non-urological symptomatology.
The long-term consequences of such obstruction including
impaired renal function and infection, remain serious problems for these
patients despite newer methods of diagnosis and treatment (13). Because of the
threat of premature death, early diagnosis and appropriate management are
imperative. All infants or older children with urinary tract infections or
abnormal voiding stream should benefit from
22
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
appropriate radiological investigations. Renal dysplasia and
renal failure are the primary causes of death in neonates with PUV who survive
initial pulmonary problems (23).
According to CHURCHILL B.M. (24) the treatment of PUV is now
in its fourth major phase. The first phase was recognition of the entity. The
second was treatment but in which the mortality rate, particularly in neonates
in the first month was almost 50 percent in most major series. The third phase
consisted of markedly improved survival rates, in which the mortality rate in
most tertiary paediatric urology centres was less than 10 percent. In the
fourth phase the challenge after having kept these children alive is to get
optimal renal function, so that optimal growth and the late complications of
dialysis and transplantation are avoided.
It is in this light that we reviewed the files of 28 patients
with PUV followed up in the University Teaching Hospital, Central Hospital and
the General Hospital in Yaoundé over an 11 year period (1st January 1985
to 31st December 1996) to assess the diagnostic methodology and outcome.
To the best of our knowledge, no study has been done with
these objectives in Cameroon. We hope to come out with pertinent findings and
recommendations which will help physicians make early diagnosis and institute
appropriate management thus avoiding long-term renal compromise and death from
this disorder.
23
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
OBJECTIVES
24
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
a) GENERAL OBJECTIVES
* To make a global assessment of PIN in Yaoundé
b) SPECIFIC OBJECTIVES
* To review the epidemiological aspects of PIN in
Yaoundé.
* To assess the clinical presentation of PIN in
Yaoundé.
* To appraise the diagnostic procedures.
* To assess the outcome of the patients following surgery (in
terms
of renal function, patient growth, urinary tract abnormalities,
dialysis and transplantation).
25
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|

REVIEW OF LITERATURE
26
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
A. ANATOMY OF THE NORMAL URETHRA (Figs. 1, 2)
(25)
The male urethra extends from the bladder neck to the meatus
at the tip of the penis. It has two portions, anterior and posterior. The
anterior urethra (15cm) comprises the bulbous and penile portions whereas the
posterior consists of the first few centimetres distal to the bladder neck and
includes the prostatic urethra (3cm) and the membranous urethra (18mm) where
the external sphincter resides. Located in the posterior mid-position of the
prostatic urethra is an elevation called the verumontanum (coliculus seminalis)
containing the paired ejaculatory duct openings. On its surface in the midline
is the opening of the utriculus prostaticus, the rudimentary homologue of the
uterus in the male.
Extending inferiorly from the verumontanum in the midline is
the crista urethralis, and diverging from this are the plicae colliculi, which
merge into the external folds. These plicae may be normal remnants of the
terminal wolfian ducts which regress during embryogenesis, leaving only the
ejaculatory duct openings.
B. EMBRYOLOGY OF THE URINARY SYSTEM
(26)
Functionally, the urogenital system can be divided into two
entirely different components (1) The urinary system and (2) the genital
system.
Embryologically and anatomically however, they are intimately
interwoven Both develop from a common mesodermal ridge along the posterior wall
of the abdominal cavity, and the excretory ducts of both systems, initially
enter a common cavity, the cloaca.
With further development, the overlapping of the two Systems
is particularly evident in the male. The primitive excretory duct first
functions as a urinary duct but later is transformed into the main genital duct
Moreover , in the adult the urinary as well as the genital organs discharge
urine and semen through a common duct, the penile urethra.
27
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
1.1 THE KIDNEY SYSTEMS
Three different, slightly overlapping kidneys are formed
during intra -uterine life in man: the PRONEPHROS, the MESONEPHROS, and
METANEPHROS or permanent kidney.
PRONEPHROS: In the human embryo the
pronephros is represented by a 7 to 10 solid cell groups in the cervical region
( Fig. 3B) The first formed vestigial nephrotomes regress before the last ones
are formed, and at the end of the fourth week all indications of the pronephric
system have disappeared.
*MESONEPHROS: During regression of
the pronephric system, the first excretory tubules of the mesonephros appear.
They lengthen rapidly, form an «S» - shaped loop, and acquire a
glomerulus at their medial extremity. Here the tubule forms the Bowman's
capsule.
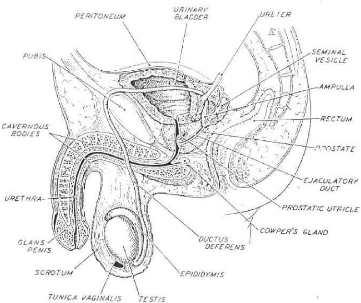
Fig. 1 Diagrammatic representation of the male genital system.
The midline structures are shown in a sagittal section;
bilateral structures, such as testis, epididymis, vas deferens,
and seminal vesicle, are depicted intact.
(From Bloom/Fawcett 28
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
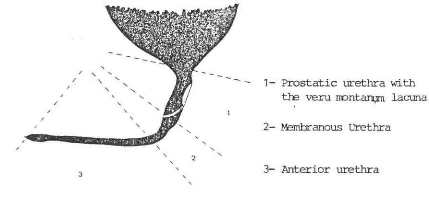
(6))
Fig. 2 The normal urethra (from Baunin et al
(5))
The capsule and glomerulus form together a mesonephric (renal)
corpuscle - At the opposite end the tubule enters the longitudinal collecting
duct known as the mesonephric or Wolfian duct (fig 3).
In the middle of the second month, the mesonephros forms a
large ovoid organ on each side of the midline. Since the developing gonad is
located on its medial side the ridge formed by both organs is known as the
urogenital ridge. While the caudal tubules are still differentiating the
cranial tubules and glomeruli show degenerative changes and by the end of the
second month, the majority has disappeared. A few of the caudal tubules and the
mesonephric duct however persist in the male but disappear in the female.
Although great resemblances in ultra structure exist between the mesonephros
and metanephros, functional activity of the mesonephros has not been
demonstrated in the human embryo.
*METANEPHROS OR PERMANENT KIDNEY:
The third urinary organ, the metanephros or permanent kidney appears in the
fifth week. Its excretory units develop from the metanephric mesoderm (fig 4)
in the same manner as in the mesonephric system.
29
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
THE COLLECTING SYSTEM
The collecting ducts of the permanent kidneys develop from the
ureteric bud, an outgrowth of mesonephric duct close to its entrance into the
cloaca (fig 4). The bud penetrates the metanephric tissue, which, as a cap is
moulded over its distal end (Fig. 4) Subsequently the bud dilates forming the
primitive renal pelvis; simultaneously it splits into a caudal portion, the
future major calyces. Each calyx, while penetrating into the metanephric
tissue, forms two new buds. These buds continue to subdivide until 12 or more
generations of the tubules have been formed. While at the periphery more
tubules are formed until the end of the fifth month, the tubules of the second
order enlarge and absorb those of the third and fourth generations, thus
forming the minor calyces of the renal pelvis. During further development, the
collecting tubules of the fifth and successive generations elongate
considerably and converge on the minor calyx, thereby forming the renal
pyramid. Hence , the ureteric bud gives rise to the ureter, renal pelvis, the
major and minor calyces and approximately one to three million collecting
tubules.
THE EXCRETORY SYSTEM
Each newly formed collecting tubule is covered at its distal
end by a so-called metanephric tissue cap. Under the inductive influence of the
tubule cells of the tissue cap form small vesicles, the renal vesicles which in
turn give rise to small tubules. These tubules form the nephrons or excretory
units. The proximal end of the nephron forms the Bowman's capsule of the renal
glomerulus. The distal end forms an open connection with one of the collecting
tubules, thus establishing a passageway from the glomerulus to the collecting
unit. Continuous lengthening of the excretory tubule results in the formation
of the proximal convoluted tubule, the loop of Henle, and the distal convoluted
tubule.
30
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
EMBRYOLOGY OF THE UROGENITAL SYSTEM (FROM LANGMAN
(49)
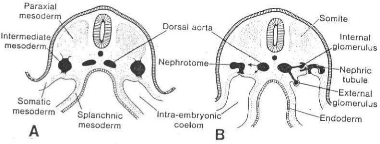
Fig.3a Schematic transverse sections through embryos at
various stages of development to show the formation of the nephric tubule.
A, At 21 days; B, at 25 days. Note the formation Of the
external and internal glomeruli, and the open connection between the coelomic
cavity and the nephric tubule (modified after Heuser).
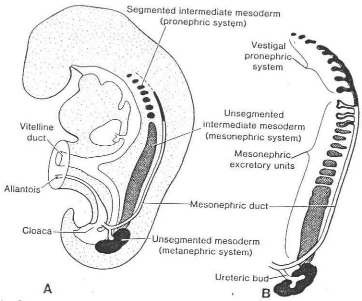
Fig. 3b A, Schematic diagram showing the
relation of the intermediate mesoderm of the pronephric, mesonephric, and
metanephric systems. In the cervical and upper thoracic regions the
intermediate mesoderm is segmented; in the lower thoracic, lumbar, and sacral
regions it forms a solid, unsegmented mass of tissue, the nephrogenic cord.
Note the longitudinal collecting duct, initially formed by the pronephros but
later taken over by the mesonephros. B, Schematic representation of
the excretory tubules of the pronephric and mesonephric systems in a
five-week-old embryo. Note the remnant of the pronephric excretory tubules and
longitudinal collecting duct. 31
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
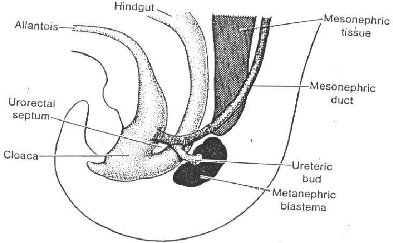
Fig 4 Schematic drawing to show the relationship of the
hindgut and cloaca at the end of the fifth week. The
ureteric bud begins metanephric mesoderm or blastema.
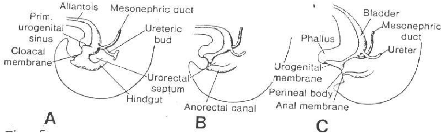
Fig. 5: Diagrams showing the division of the cloaca into
the urogenital sinus and anorectal canal. Note that the mesonephric duct is
gradually absorbed into the wall of the urogenital sinus and that the ureters
enter separately. A, End of the fifth week; B, seven weeks;
C, eight weeks.
32
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
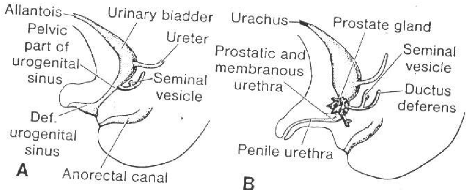
Fig 6 A, Development of the
urogenital sinus into the urinary bladder, the pelvic part of the urogenital
sinus, and the definitive urogenital sinus. B,
In the male the definitive urogenital sinus develops into the penile
urethra. The prostate gland is formed by outbuddings of the urethra, while the
seminal vesicles are formed by an outbudding of the ductus deferens.
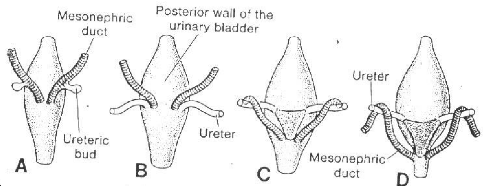
Fig 7 Dorsal view of the bladder to show the
relationship of the ureters and mesonephric ducts during development. Initially
the ureter is formed by an outgrowth of the mesonephric duct, but with time it
obtains a separate entrance into the urinary bladder. Note the trigone of the
bladder formed by incorporation of the mesonephric ducts.
33
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
Hence the kidney develops from two different sources (1) the
matanephric mesoderm which provides the excretory units and (2) the ureteric
bud which gives rise to the collecting system.
1-2 BLADDER AND URETHRA
During the fourth to seventh week of development, the
uro-urectal septum divides the cloaca into the ano-rectal canal, and the
primitive urogenital sinus (Fig.5). The cloacal membrane itself is then divided
into the urogenital membrane, anteriorly, and the anal membrane, posteriorly
(Fig.5C).
Three portions of the primitive urogenital sinus can be
distinguished: (1) the upper and largest part is the urinary bladder (Fig. 6A).
Initially the bladder is continuous with the allantois, but when the lumen of
the allantois is obliterated, a thick fibrous cord, the urachus remains,
connecting the apex of the bladder with the umbilicus. In the adult, the
ligament is known as the median umbilical ligament; (2) A rather narrow canal,
the pelvic part of the urogenital sinus; which in the male gives rise to the
prostatic and membranous parts of the urethra; (3) the definitive urogenital
sinus, also known as the phallic part of the urogenital sinus. It is
considerably flattened from side to side and is separated from the outside by
the urogenital membrane.
During division of the cloaca, the caudal portions of the
mesonephric ducts are gradually absorbed into the wall of the urinary bladder
(Fig.7). Consequently the ureters, initially outbuddings of the mesonephric
ducts, enter the bladder separately (Fig.7B). As a result of the ascent of the
kidneys, the orifices of the ureters move further cranial; those of the
mesonephric ducts more close together to enter the prostatic urethra and in the
male, become the ejaculatory ducts (Fig.7C, D). Since both the mesonephric
ducts and the ureters are of mesodermal origin, the mucosa of the bladder
formed by incorporation of the ducts, the trigone of the bladder is of
mesodermal origin. The remaining part of the bladder is derived from the
urogenital sinus and is endodermal in origin. With time, the mesodermal lining
of the trigone is replaced by endodermal epithelium so that finally the inside
of the bladder is completely lined with epithelium of endodermal origin. 34
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
The epithelium of the male and female urethra is of endodermal
origin, while the surrounding connective and smooth muscle tissue are derived
from the splanchnic mesoderm. At the end of the third month, the epithelium of
the prostatic urethra begins to proliferate and forms a number of outbuddings
which penetrate the surrounding mesenchyme. In the male, these buds form the
prostatic gland (Fig.6 B). In the female, the cranial part of the urethra gives
rise to the urethral and paraurethral glands.
C. EMBRYOGENESIS OF POSTERIOR URETHRAL VALVES
(11,13)
Currently, the most accepted view is that type I valves arise
from the urethrovaginal folds which become the plicae colliculi in the course
of development. The origin of the urethrovaginal folds is a matter of dispute.
Some authors believe that these folds represent the fibrous track left behind
by the Wolfian ducts as they migrate posteriorly and medially around the wall
of the urogenital sinus until they meet the paramesonephric duct at the
müllerian tubercle in the middling, whereas others think they represent
the anterior potion of the hymenal ring and thus would be müllerian
derivatives.
However abnormal formation or regression of these plicae
colliculi may be involved in the genesis of typical PUV which are exaggerations
of the normal folds. Type III membranes may be variable in origin. Some may
represent type I valves with marked anterior fusion, whereas others may
represent incomplete disappearance of the urogenital membrane.
A genetic component has been postulated from occasional
observations of PUV in twin and no-twin siblings (8, 11, 27, and 16). But the
genetic factors in the pathogenesis of PUV are poorly understood. PUV have also
been described to be associated with other chromosomal abnormalities ad Down's
Syndrome (19, 26).
35
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
D. CLASSIFICATION OF POSTERIOR URETHRAL VALVES
LANGENBECK in 1802 is credited with the first description of
PUV, but in 1919 YOUNG H.H, FRONTZ W.A and BALDWIN J.C. reported 36 cases, 12
from a personal series and a further 24 from the world literature (cited by
DINNEEN and Duffy in 10). YOUNG et al described 3 types of valves -Fig.8 (cited
in 28, 29, 30, 11, 12, 2, 13, and 31)
Type I: It is a bicuspid valve that
originates just distal to the verumontanum on the floor of the posterior
urethra and diverges distally in an antero-lateral orientation to fuse
anteriorly in the midline at the twelve o'clock position at the anterior wall
just proximal to the membranes urethra. The appearance endoscopically is that
of two membranes, paired in a manner similar to the vocal cords, fused
anteriorly. The fusion creates a valve which obstructs the outflow of urine
while allowing the retrograde passage of catheters or irrigating fluid 95% of
PUV are Type I, with variations in leaflet thickness and in
the degree of coalescence at the twelve o'clock position. The resulting
obstruction consists of filmy membranes which are easily disrupted, or at
worst, a thickened tissue with a small inferior opening. This type corresponds
to the "Spinnaker Sail" appearance.
Type II: They are a series of folds
that run between the verumontanum and the bladder neck and probably are
non-obstructive.
Type III: They are obstructing
diaphragms with a central opening, located in the membranous urethra. They do
not have a typical attachment to the inferior portion of the verumontanum and
are of a different embryological origin. There are two subgroups:
IIIa. - below the verumontanum; and IIIb above the verumontanum.
36
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
BASIC TYPES OF POSTERIOR URETHRAL VALVES (FROM GARRY S.H.
(30))
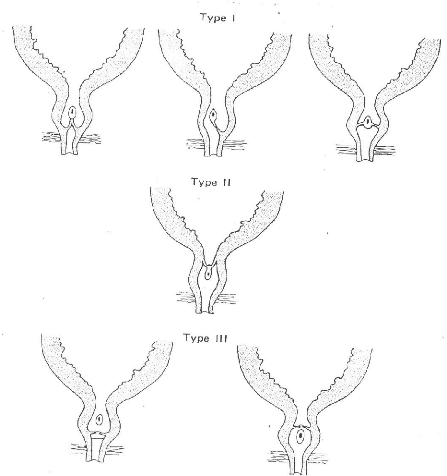
The YOUNG's classification however, developed largely on the
basis of autopsy dissection before the advent of endoscopy, has some
inaccuracies and does not correspond well to modern ideas of the normal anatomy
and embryology of this region (11). 37
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
The three types of valves described in YOUNG's series had
undergone urethral manipulation before assessment and so this classification
has been criticized (10).
This misconception appears to result from post-mortem
dissections from the anterior approach, cutting through the fused anterior
portion of a structure that modern endoscopic studies reveal is actually a
diaphragm with a lumen, making it appear as if there were two leaflets instead
of a membrane (11).
In a similar fashion, there are superficial fibroelastic
bundles that pass upward and laterally towards the base of the bladder. These
are the structures that YOUNG et al thought correspond to Type II valves.
Although they are occasionally quite prominent, most authors agree that these
structures are almost never obstructive (11). Current endoscopic evidence (29,
30, 11) dismiss the existence of Type II valves.
According to DEWAN (29), the acceptance of a single basic
morphology for the posterior urethral pathology suggests that there is only one
embryological process, which is probably a persistence of the normal attachment
of the verumontanum to the posterior urethra. He has thus proposed a
nomenclature for valves different from YOUNG's classification. The type III
valve which is a more distal bulbar obstructing membrane with a central hole
may well constitute a persistence of the urogenital diaphragm and referred to
as COBB's COLLAR, whereas the term COPUM (Congenital Obstructive Posterior
Urethral Membrane) may be appropriate for the posterior urethral valves (Type
I).
E. PATHOPHYSIOLOGIC CHANGES INDUCED BY PUV ON THE URO-
GENITAL TRACT(32, 10, 11, 33, 64, 17, 35, 36)
i. Urethral Changes
Proximal to the obstruction, the urethral dilates and
balloons. A proximal diverticulum may develop and dilatation and gaping of the
prostatic and ejaculation ducts may occur. PUV have been mentioned as a
possible cause of urethro-ejaculatory
reflux of infected or sterile urine (with possibility of
bilateral obstruction of the genital
38
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
tract) and presumed to be a possible cause of acute
epididymitis and infertility. Under normal circumstances, the reflux of urine
into the male genital tract is impossible. The anti-reflux mechanism is
effected by the oblique passage of the ejaculatory duct through the thick
prostatic tissue. This mechanism might be rendered incompetent by the extreme
attenuation of the foetal prostatic tissue consequent upon excessive dilatation
of the obstructed prostatic urethra. It should be noted that the foetal kidneys
start producing urine during the third month of gestation would prevent the
growth of the prostate; early urethral obstruction would prevent the growth of
the prostrate and the development of a competent anti-reflux mechanism.
Infertility could also result from retrograde ejaculations following surgery on
the bladder neck.
ii. Vesical Changes
Early, the detrusor and trigonal thickening and hypertrophy
compensate for the outlet obstruction and lead to complete bladder emptying.
This change leads to progressive development of the bladder trabeculation,
cellules then diverticula. Beyond a certain phase, bladder decompensation
occurs and is characterized by the above changes, pins variable amounts of
residual urine. Trigonal hypertrophy leads to secondary ureteral obstruction
due to increased resistance to flow through the intravesical ureter. With the
detrusor decompensation and residual urine accumulation, there is stretching of
the hypertrophied trigone, which increases ureteral obstruction. This is the
mechanism of back pressure on the kidneys in the presence of vesical outlet
obstruction, while the uretero-vesical junction maintains its competence.
Catheter drainage of the bladder relieves trigonal stretch and improves
drainage from the upper tract. A very late change with persistent obstruction
(more frequently encountered with neurogenic dysfunction) is decompensation of
the uretero-vesical junction, leading to reflux, which aggravates the back
pressure in the upper tract by exposing it to abnormally high intravesical
pressure, in addition to favouring (the onset or persistence ) of urinary tract
infection. Should ureteral obstruction be unilateral a compensatory hypertrophy
of the contralateral kidney will develop. Total renal function therefore
remains normal.
39
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
Bladder diverticula and unilateral vesicoureteral reflux may
serve as 'popoff' mechanisms to buffer high pressures in the urinary tract.
Patients with PUV may also develop valve bladders, which are thick walled,
poorly compliant and often with high resting pressures even at small urine
volumes. It is this high bladder pressure that is so damaging to the urinary
tract. Bladder dysfunction (unstable, poorly compliant and over distended
bladders which are variations of the same basic urodynamic pattern) that
changes with time towards decompensation is clearly a contributory factor to
urinary incontinence.
iii. Ureteral Changes
The first noted change is gradual progression in ureteral
distension. This increases ureteral wall stretch, which in turn increases
contractile power and ureteral hyperactivity and hypertrophy develops. Because
the ureteral musculature runs in an irregular helical pattern, stretching of
its muscular elements leads to lengthening as well as widening. This is the
start of ureteral decompensation, where tortousity and dilatation become
apparent. These changes can progress, leading to marked ureteral dilatation and
lengthening, and the ureter becomes atonic with infrequent or completely absent
peristalsis.
iv. Pelvicalyceal Changes
The renal pelvis and calyces being subjected to progressively
increasing volumes of retained urine progressively distend. The pelvis first
shows evidence of hyperactivity and hypertrophy and then progressive dilatation
and atony. The calyces show the same changes to a variable degree depending on
whether the renal pelvis is intra or extra-renal. In the latter, the calyceal
dilatation may be minimal in spite of marked pelvic dilatation. In the intra
-renal pelvis, calyceal dilatation and renal parenchymal damage are maximum.
The successive phases seen with obstruction are rounding of the fornices,
followed by flattening of the papillae and finally clubbing of the minor
calyces.
In neonates and infants there may be extravasation of urine at
the level of the renal pelvis or the ureterovesical junction with formation of
urinary ascitis and perirenal
40
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
and retroperitoneal urinomas resulting in abdominal distension
v. Renal Parenchymal changes
Since urine formation begins between the ninth and twelfth
weeks of gestation corresponding to the formation of the inner cortical
nephrons in the centrifugally developing kidney , obstruction to urinary
outflow could increase hydrostatic pressure and thus affect the environment of
the foetal kidney during the very early phases of morphogenesis, resulting in
hypoplastic or dysplastic renal development in addition to simple
hydronephrosis whereas obstruction late in gestation may produce simple
hydronephrosis It should be noted that nephron differentiation occurs up to the
thirty-second week of gestation.
With progressive pelvicalyceal distension, there is
parenchymal compression against the renal capsule. This, plus the more
important factor of compression of the arcuate vessels as a result of the
expanding distended calyces results in a marked drop in renal blood flow. This
phenomenon leads to progressive parenchymal compression and ischemic atrophy.
Lateral groups of nephrons are affected more than central ones, leading to
patchy atrophy with variable degree of severity. The glomeruli and proximal
convoluted tubules suffer most, of this ischemia. Associated with the increased
intrapelvic pressure there is progressive dilatation of the collecting and
distal tubules with compression and atrophy of tubular cells.
Whereas dilation of the calices and the thinness of the
parenchyma my be explained on the bases of atrophy from back pressure of
obstruction and reflux, the etiology of variants such as asymmetrical kidney
morphologies, the occurrence of near normal renal parenchyma in some kidneys
exhibiting all the ureteral and caliceal stigmas of severe obstruction and
dysplasia, is not the same.
HENNEBERY and STEPHENS (37) have clearly demonstrated that
these variants may be due to ectopic origins of the ureteral buds from the most
caudal part of the Wolfian duct, which leads to induction of defective or
sparse mesenchyme of the tail end of the nephrogenic cord with resultant
dysplasia and hypoplasia, respectively. The key to the potential quality of the
renal parenchyma is the ureteral orifice. This is the "bud theory" of the renal
morphology 41
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
42
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
MATERIALS AND METHODS
43
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
1. PLACE OF STUDY
The study was carried out in the University Teaching Hospital,
Central Hospital and the General Hospital in Yaounde.
2. TIME OF STUDY
We reviewed the files of 28 patients treated or followed up
for PUV from 1st January 1985 to the 31st of December 1996 (11
years). As from 1st January 1997 to 1st July 1997 we sent
out messages (by mail, phone calls) to 22 patients who were alive. They were
requested to come for evaluation. This constituted the prospective phase
3. PATIENT SELECTION
28 patients were recruited in the study. These patients had
been treated for PUV in the above health institutions or elsewhere. Of the 28
patients, 6 died, 10 lost to follow-up and 12 reassessed in the prospective
phase. Those who were not seen in the prospective phase were considered lost to
follow-up.
4. STUDY DESIGN
The study was carried out in two phases a retrospective
cross-sectional and a prospective longitudinal descriptive review of clinical
data. In the retrospective cross-sectional phase we noted the history,
diagnostic procedures, treatment and follow-up parameters (stream, height,
weight, BUN, creatinine, urine cultures and post-operative complications) and
in the prospective phase we insisted on seeing the patients at least monthly
for re-assessment of the above follow-up parameters and control ultrasound and
cystourethrograms requested.
A questionnaire was filled for each patient.
5. ETHICS
Consent was obtained from all the parents before admission
into the study. Explanations as to the innocuity of the study were given as
well as benefits incurred from regular follow-up after surgery to avoid short
and long-term complications. 44
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
6. DATA ANALYSIS
The data collected was analysed in a computer (type NCR) using
the Epi-lnfo medical software.
45
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|

RESULTS
46
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
Table 1: CLASSIFICATION OF ALL THE PATIENTS
ACCORDING TO AGE AT DIAGNOSIS
|
GROUP I
(Age < 1 month)
|
GROUP III
(1 to 12 months)
|
GROUP III
(> 12 months)
|
|
Number / %
|
5 (18%)
|
9 (32%)
|
14 (50%)
|
|
Mean age
|
0.5 months
|
4.6 months
|
66 months (5.5 years)
|
|
(range 9 - 21 days)
(0.3 - 0.7 months)
|
(range 1 - 10 months)
|
(range 13 - 156 months)
|
Table 2: PAST HISTORY
|
Number
|
%
|
|
UTI
|
14
|
50
|
|
Hypertension
|
2
|
7
|
|
Renal disease
|
1
|
3.6
|
|
Cryptochidism
|
1
|
3.6
|
|
G6PD *
|
1
|
3.6
|
|
Strabismus
|
1
|
3.6
|
|
IVSD**
|
1
|
3.6
|
*glucose 6 phosphate dehydrogenase deficiency **intra ventricular
septal defect
47
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
Table 3: PRESENTING COMPLAINTS AT DIAGNOSIS
ACCORDING TO AGE GROUPS (N=28)
|
|
I (N=5)
|
II (N=9)
|
III (N =14)
|
TOTAL (%)
|
|
A)
|
URINARY SYMPTOMS
|
|
|
|
|
|
Dribbling
|
2
|
5
|
10
|
17(60.7)
|
|
Dysuria
|
1
|
7
|
7
|
15 (54)
|
|
Urine retention
|
1
|
2
|
4
|
7 (25)
|
|
Chronic renal failure
|
1
|
1
|
2
|
4 (14)
|
|
Hematuria
|
-
|
1
|
3
|
4 (14)
|
|
Pollakiuria
|
-
|
1
|
1
|
2 (7)
|
|
Pyuria
|
-
|
1
|
1
|
2 (7)
|
|
Oedemato-ascitic syndrome (urinary ascitis)
|
1
|
-
|
-
|
2 (7)
|
|
Hypogastric pain
|
-
|
1
|
1
|
1 (3.6)
|
|
Paraphimosis
|
-
|
-
|
-
|
1 (3.6)
|
|
Incontinence
|
-
|
1
|
1
|
1 (3.6)
|
|
|
B)
|
NON-URINARY SYMPTOMS
|
|
|
|
|
|
Fever
|
1
|
4
|
2
|
7 (25)
|
|
Failure to thrive
|
1
|
4
|
2
|
7.(25)
|
|
Vomiting
|
-
|
2
|
1
|
3 (10.7)
|
|
Diarrhoea
|
-
|
3
|
-
|
3 (10.7)
|
|
Dehydration
|
-
|
2
|
1
|
3 (10.7)
|
|
Anorexia
|
1
|
2
|
-
|
3 (10.7)
|
|
Respiratory distress
|
1
|
-
|
1
|
2 (7%)
|
|
Abdominal distension
|
1
|
-
|
1
|
2 (7%)
|
|
Polydypsia
|
-
|
-
|
1
|
1 (3.6)
|
48
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
Table 4: MAIN PHYSICAL FINDINGS AT DIAGNOSIS
ACCORDING TO AGE GROUPS (N=28)
|
I (N = 5)
|
II (N = 9)
|
III (N = 14)
|
TOTAL (%)
|
|
Umblical hernia
|
3
|
1
|
2
|
6 (21)
|
|
Bladder distention
|
1
|
-
|
2
|
3 (10.7)
|
|
I V S D
|
2
|
-
|
-
|
2 (7)
|
|
Hepatomegally
|
1
|
-
|
1
|
2 (7)
|
|
Bilateral flank masses
|
-
|
1
|
1
|
2 (7)
|
|
Oedemato-ascitic syndrome
|
1
|
-
|
1
|
2 (7)
|
|
Hydrocoele
|
-
|
2
|
-
|
2 (7)
|
|
Trisomy 21
|
1
|
-
|
-
|
1 (3.6)
|
|
Bilateral inguinal hernia
|
1
|
-
|
-
|
1 (3.6)
|
|
Paraphimosis
|
-
|
1
|
-
|
1 (3.6)
|
|
Splenomegally
|
1
|
-
|
-
|
1 (3.6)
|
|
Neonatal jaundice
|
1
|
-
|
-
|
1 (3.6)
|
Table 5: BIOLOGIC INVESTIGATIONS AT DIAGNOSIS
|
I (N° + %)
|
II (N° + %)
|
III (N° + %)
|
TOTAL
|
|
CBC (19)
|
|
|
|
|
|
WBC = 10.000 (12)
|
3 (25)
|
6 (50)
|
3 (25)
|
12
|
|
Hb = 11 g / 1 (15)
|
3 (20)
|
6 (40)
|
6 (40)
|
15
|
|
BUN (21)
> 45 mg % (12)
|
1 (8.3)
|
2 (16.7)
|
9 (75)
|
12
|
|
Creatinine (22)
> 1.5 mg % (15)
|
1 (6)
|
6 (40)
|
8 (53)
|
15
|
|
K+ (9)
> 5 mEq / 1 (5)
|
2 (40)
|
2 (40)
|
1 (20)
|
5
|
49
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
Table 6: GLOMERULAR FILTRATION RATES AT
DIAGNOSIS
|
I
|
II
|
III
|
|
Mean ages (in years)
|
0.04
|
0.38
|
5.5
|
|
Mean weights (in kg)
|
3.6
|
7
|
21
|
|
Mean Creatinine (mg %)
|
1.5
|
1
|
12
|
|
Mean GFR (ml / min/1.73m2)
|
5
|
14
|
19
|
GFR (ml / min / 1.73m2) was calculated from
COCKCROFT'S formula:
|
= 140 -age (yrs) X weight (kg) 72 x Creatinine (mg
%)
* Nephron 16: 31 - 71, 1976
|
Table 7: RESULTS OF URINE CULTURE AT DIAGNOSIS (N =
12)
|
GERM
|
N°
|
%
|
|
E. coli
|
5
|
26
|
|
Psuedomonas aeroginosa
|
2
|
11
|
|
Moraxella spp
|
2
|
11
|
|
Klebsiella pneumoniae
|
2
|
11
|
|
Enterobacter aerogenes
|
1
|
5
|
|
Proteus mirabilis
|
1
|
5
|
|
TOTAL
|
13
|
100
|
Urine cultures were available in 19 patients. In 12 they were
positive of the above pathogens and sterile in 7. One patient had both a
klebsiella pnuemoniae and Proteus
mirabilis infection.
50
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
Table 8: MAIN ULTRA SONOGRAPHIC FINDINGS AT
DIAGNOSIS (N = 18)
|
N°
|
%
|
|
Bilateral ureterohydronephrosis
|
17
|
94
|
|
Trabeculated multidiverticular bladder
|
12
|
67
|
|
Dilated posterior urethra
|
13
|
72
|
Other associated findings:
+ Bilateral renal cortical atrophy in 3 (17%)
+ Bilateral renal antrophy in 1 (6%)
+ Bilateral renal parenchyma atrophy and megaureters in 1 (6%) +
Megaureters with the left ectopic (6%)
Table 9: MAIN VOIDING CYSTOURETHROGRAM
FINDINGS
AT DIAGNOSIS (N = 18)
|
N°
|
%
|
|
VUR
|
5
|
28
|
|
Trabeculated multidiverticular bladder
|
14
|
78
|
|
Dilated posterior urethra
|
15
|
83
|
|
Presence of valves
|
7
|
39
|
VUR was bilateral in 4 patients and on the right in 1 patient.
Large urine residual volumes were present in 2 patients. Hutch's diverticulum
was noted in 2.
Table 10: IVP FINDINGS AT DIAGNOSIS (N = 6)
|
N°
|
%
|
|
Bilateral uretero-hydronephrosis
|
5
|
83
|
|
Bilateral late secretion
|
1
|
17
|
|
UPJ obstruction
|
1
|
17
|
|
Left non --functional kidney
|
1
|
17
|
|
Partial Pelvic duplication
|
1
|
17
|
51
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
|
A 9 months old boy showing a dilated posterior urethra and a
PUV.
|
52
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
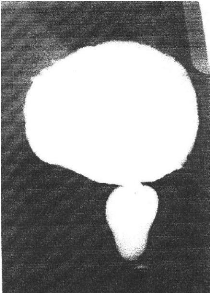
Voiding cystourethrogram of a 9 day old child showing a large
trabeculated bladder, dilated
posterior urethra and a filling defect at the posterior
urethra indicating a PUV.
|
An 8 year old with a large trabeculated multidivertucular
bladder.
|
|
A 2 months old with bilateral passive reflux and megaureters
|
53
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
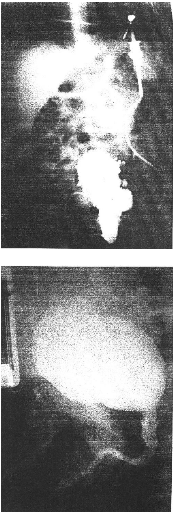
A 3 months old with a multidiverticular bladder and a dilated
posterior urethra
Control cysto urethrogram in an 8 year old boy following
resection of PUV at the age of 2 years showing a normal bladder wall and
posterior urethra.
4

Table 11: SCINTIGRAPHY (N = 2)
|
N°
|
%
|
|
Bilateral renal atrophy with poor uptake of DMSA
|
1
|
50
|
|
Left renal atrophy
|
1
|
50
|
NB There was a strong suspicion of dysplasia in the patient with
bilateral atrophy.
Table 12: URODYNAMIC STUDIES (N = 2)
|
N°
|
%
|
|
Normal
|
1
|
50
|
|
Reduced bladder compliance
|
1
|
50
|
Table 13: SURGICAL TREATMENT (N = 25)
|
TYPE
|
N°
|
%
|
|
Endoscopic resection
|
20
|
84
|
|
BLOCKSOM vesicostomy
|
6
|
24
|
|
Cystostomy
|
3
|
12
|
|
Catheter ablation
|
2
|
8
|

2 patients were lost to follow-up after diagnosis and didn't
undergo and surgery. One patient with terminal renal failure underwent
ureterostomy in France but was lost to follow-up.
22 patients underwent surgery in Cameroon, 1 in Britain and 1 in
France.
5 Patients did not have endoscopic resection. 4 in the
vesicostomy group (2 died and 2 pending by the end of the study) and 1 in the
catheter ablation group.
55
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
Table 14: SECONDARY PROCEDURES IN 11 PATIENTS
(N=15)
|
N°
|
%
|
|
Ureteroplasty + re-implantation
|
4
|
36
|
|
Nephrostomy
|
3
|
27
|
|
Circumcision
|
3
|
27
|
|
Ureterostomy
|
2
|
18
|
|
Diverticulectomy
|
2
|
11
|
|
Urethrostomy
|
1
|
9
|
Table 15: OUTCOME OF THE 28 PATIENTS
|
N°
|
%
|
|
Deaths
|
6
|
21
|
|
Re-evaluation prospectively
|
12
|
43
|
|
Lost to follow-up
|
10
|
36
|
|
Incontinence
|
8
|
29
|
Table 16: CAUSES OF DEATHS
|
N°
|
%
|
|
Septicemia
|
3
|
50
|
|
Post-obstructive diuresis
|
2
|
33
|
|
Chronic renal failure
|
1
|
17
|
1 case of septicaemia was associated with congestive heart
failure.
56
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
Table 17: EVALUATION OF RENAL FUNCTION AND WEIGHTS AT
DIAGNOSIS AND AT FINAL FOLLOW-UP PRE-OPERATIVELY:
|
Age
|
Weight
|
BUN
(mg%)
|
Creatinine
(mg%)
|
GFR
ml/min/1.73m2
|
|
A.N
|
23 months
|
12.5
|
1.8
|
1
|
24
|
|
D.Y
|
16 months
|
10
|
50
|
1.1
|
18
|
|
E.N
|
3.5 months
|
4.22
|
22
|
1.2
|
7
|
|
F.K
|
10 years
|
33.5
|
32
|
0.8
|
76
|
|
J.B
|
2 years
|
12
|
42
|
1.8
|
13
|
|
K.K.E
|
2.5 years
|
12.8
|
14
|
0.4
|
61
|
|
N.J
|
11 months
|
6.1
|
-
|
0.8
|
15
|
|
N.C
|
14 months
|
9.5
|
36
|
0.4
|
46
|
|
N.M
|
3 weeks
|
3.47
|
73
|
1.6
|
4
|
POST-OPERATIVELY(at end of
follow-up):
|
Age
|
Weight
|
BUN
(mg%)
|
Creatinine
(mg%)
|
GFR
ml/min/1.73m2
|
|
A.N
|
11 yrs 11months
|
32.5
|
-
|
1
|
58
|
|
D.Y
|
9 years
|
23
|
69
|
1.1
|
38
|
|
E.N
|
14 months
|
9.980
|
23
|
0.58
|
33
|
|
F.K
|
12.5 years
|
37.8
|
13
|
0.6
|
112
|
|
J.B
|
9 years
|
27
|
62
|
1.8
|
20
|
|
K.K.E
|
9 years
|
25.4
|
34
|
1.3
|
36
|
|
N.J
|
2 years
|
10.8
|
91
|
1.4
|
15
|
|
N.C
|
6 years
|
19.4
|
38
|
1.2
|
30
|
|
N.M
|
6 years
|
19.8
|
29
|
0.4
|
92
|
= 140 -age (yrs) X weight (kg) 72 x Creatinine (mg
%)
GFR was calculated from COCKCROFT'S formula* GFR
(ml/min/1.73m2)
Normal values:
New born (day 1) - 5-50 ml/min/1.73m2 (mean 18
ml/min/1.73m2)
(day 6) * 15 - 90 ml/min/1.73m2 (mean 36
ml/min/1.73m2)
Older children and adults (levels reached at 6 months
* Males: 85 - 125 ml/min/1.73m2
* Females: 75 - 115 ml/min/1.73m2
> Nephron 16: - 31 -71, 1976 57
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
58
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
59
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
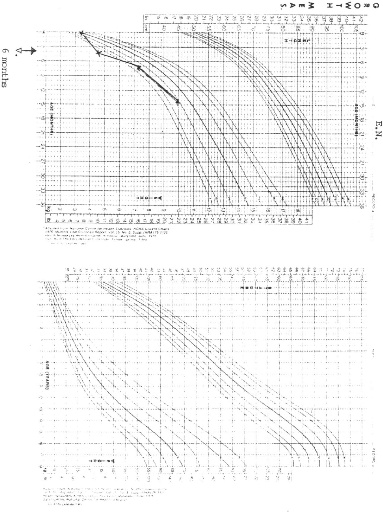
60
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
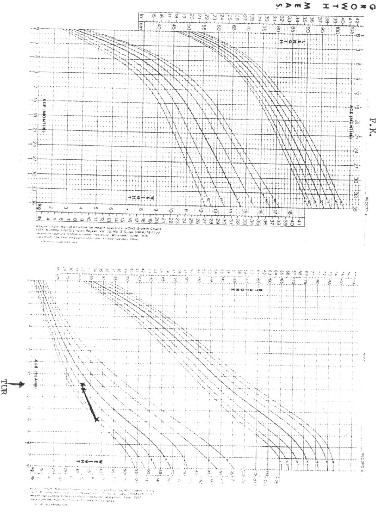
61
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
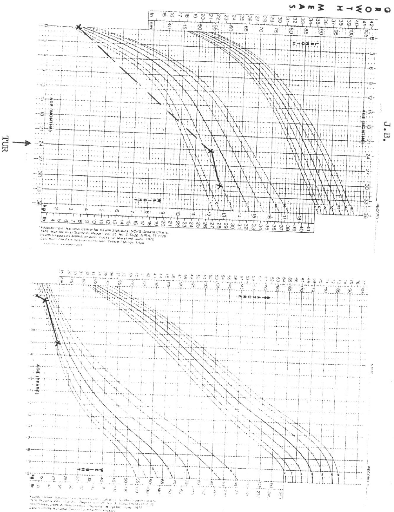
62
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
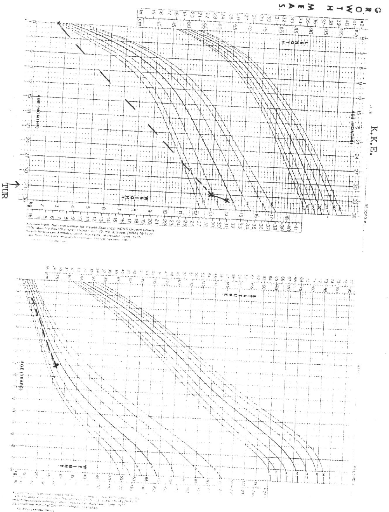
63
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
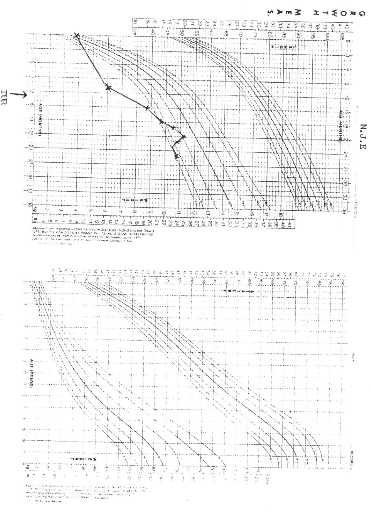
64
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
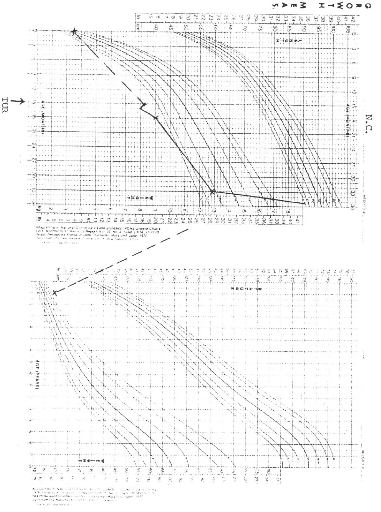
65
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
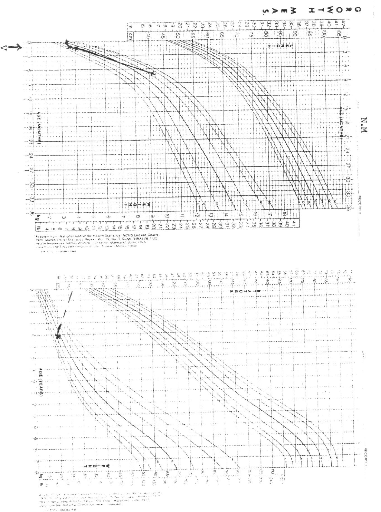
66
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|

DISCUSSION
67
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
I. EPIDEMIOLOGIC FACTORS
AGE:
The mean age at diagnosis of all the 28 patients was 2.9 years
(range 9 days to 13 years ) which is significantly higher than that reported by
COULIBALY, 2 years (18). In a series of 100 cases reported by SMITH et al (31),
42% of PUV were diagnosed before 1 month, and 56% before 1 year. In our series
50% of PUV were diagnosed after 12 months. The mean age at diagnosis in the
series reported by WARSHAW et al (38) was 39 days.
The mean age of the first consultation after onset of symptoms
was 1.6 years (range 1 day to 8 years). The mean interval between age of first
consultation and age of diagnosis was 9.7 months which is still higher than 51
days, reported by COULIBALY et al in Côte d'Ivoire (18). The mean number
of follow-ups was 3 (range 0 to 10)
We decided to divide our patients into 3 groups (see Table 1)
because it is believed that chronological age at presentation is a relatively
well -defined marker for delineating severity (14).
PAST HISTORY (Table 2):
UTI was noted in 50% of our patients. LOTTMAN (52)
noted it in 64 % of his cases, ATWEL (4) in 34% and COULIBALY (18) in 85%
of their cases. Hypertension was noted in 2 patients (7%) who presented with
end-stage renal failure. In 1 patient there was a family history of renal
disease. The cousin had end -stage renal failure from PUV, underwent kidney
transplant and is currently undergoing hemodialysis.
PRESENTING COMPLAINTS (Table 3):
In our series an abnormal urinary stream with dribbling was
most frequent in 60.7% of the patients, followed by dysuria. ANGWAFO et al (5)
noted in 100% of their patients. ATWEL (4) noted in 17% and LOTTMAN (52) in
(32%). Dysuria was the second most frequent complaint (54%) but was the most
frequent (46%) in COULIBALY's series (18). Most mothers don't know the
characteristics of a normal
68
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
urinary stream, so it should be well described when seeking to
know about the presence or absence of dribbling. Two patients with end-stage
chronic failure were observed in our series.
One had undergone kidney transplant in France. Of the
non-urinary symptoms fever (25%) and failure to thrive (25%) were most
frequent. COULIBALY et al (18) noted respectively 22% and 11%. 32% of the
non-urinary symptoms were digestive (vomiting, diarrhoea and dehydration)
MAIN PHYSICAL FINDINGS (Table 4):
Umbilical hernias were most observed ( 21 %). This finding is
very rare in literature but it was 68.2% in the series reported by ANGWAFO et
al (5). It should be noted that our series was much larger than that of ANGWAFO
et al. Bladder distention occurred in 69.8% in COULIBALY's series and was the
most frequent physical finding. Bladder distention was the second most frequent
finding in our patients. ISVD was noted in 2 patients and diagnosed in the
neonatal period. We noted the presence of trisomy 21 associated an IVSD. The
possible association of PUV and trisomy 21 has been reported by some authors
(39). It has been suggested that infants with trisomy 21 should be screened
with ultrasonography for renal and urological abnormalities early in life
because they have increased risk of developing PUV and obstructive uropathy.
II. INVESTIGATIONS
BIOLOGIC INVESTIGATIONS (Table 5 &
7)
Leucocytosis and microcytic anaemia were noted respectively in
63% and 79% of our cases. Uraemia was noted in 12 patients (57%) with BUN
greater than 45 mg% and hyperkaliemia in 5 patients (56%).
Urine cultures were requested for in all patients but results
were available in 19. 7 cultures were sterile and of the 12 in which pathogens
were identified, E. coli was the
69
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
most frequent (26%) followed by Pseudomonas
aeroginosa (11%). In COULIBALY's series (18) E. coli was also the
most frequent pathogen followed by Pseudomonas aeroginosa, and no
pathogens in 26%. No pathogens were identified in 37% of our patients. In the
series reported by FALL et al (20) the most frequent pathogen was Pseudomonas
(50%) followed by Klebsiella (38%).
ULTRASONOGRAPHY (Table 8)
In all our patients, the mothers had undergone at least one
prenatal ultrasonographic examination, but no case was diagnosed antenatally.
Diagnosis of PUV could be made as early as 16-18 weeks of gestation (19). The
major benefit of antenatal ultrasonography is to allow early diagnosis of
urinary tract malformations before post-natal infection worsens the
prognosis3)
In the post-natal period transperineal voiding ultrasound is
non-invasive and useful in diagnosing PUV. With a posterior urethral diameter
of at least 6 mm during voiding as a criterion for transperineal ultrasound
diagnosis of obstruction, sensitivity is 100%, specificity 89% and a positive
predictive value of 89% (12).
In our series 18 patients had results of ultrasound
examinations available. Bilateral ureterohydronephrosis was noted in 94%,
trabeculated bladder wall (67%) and a dilated posterior urethra in 72% of the
patients.
In 3 of our patients, diagnosed on ultrasound in the first
month, no further radiological investigations were done because of severe
sepsis and uraemia. They all underwent emergent vesicostomies.
VOIDING CYSTOURETHROGRAMS (Table 9)
Apart from the 3 patients mentioned above, all our patients
had VCUG, but results at diagnosis were available only in 18, vesico-ureteral
reflux was present in 5 patients (28%); bilateral in 4 (22%) and unilateral (on
the right) in 1 case (6%). COULIBALY et al found 13.51 % (bilateral) and 24%
(unilateral). It is reported (11) that reflux may present in up to 60% of the
patients with PUV and that, when unilateral, it is most often
70
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
on the left and associated with a poorly functioning or
non-functioning kidney and persists despite surgery. However if on the right it
is associated with a functioning kidney and resolves in most patients (11).
Direct evidence of valves was noted in 7 patients (39%) and present as filling
defects in the posterior urethra. Hutch's diverticulum which is a paraureteral
diverticulum was noted in 2 patients. One patient developed urosepsis after a
retrograde urethrocystography and was treated.
INTRAVENOUS PYELOGRAPHY (Table 10)
This was done in 6 patients and showed bilateral
uretero-hydronephrosis in 5 (83%), late secretion in 1 (17%), non-functional
left kidney in 1 (17%). COULIBALY et al (18) found 80% of their cases with
upper tract dilatation and 20% with non-functioning kidneys.
SCINTIGRAPHY (Table 11)
Radionuclide evaluation is superior to IVP in evaluating renal
functions and gives reproducible information about total and differential renal
function, but it does not provide a sensitive and accurate illustration of the
anatomic changes in the kidney .(40). Two patients benefited from this
examination (one in France and one in Britain). In one there was strong
suspicion of bilateral renal dysplasia.
BLADDER URODYNAMICS (Table 12)
Bladder urodynamics are necessary in evaluating the
bladder-sphincter complex and will help in the diagnosis of bladder dysfunction
(41). The same two patients benefited from this examination.
Reduced bladder compliance was noted in one who had
incontinence.
III. TREATMENT (Tables 13 and 14)
Of our 28 patients, 2 were lost to follow-up immediately after
diagnosis and so did not undergo surgery. 1 patient with end-stage renal
failure had ureterostomy in France. So only 25 patients had at least one major
surgical procedure mentioned in Table 13. A
71
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
total of 20 endoscopic resections were done, 1 in Britain and 1
in France.
BLOCKSOM vesicostomies were done in 6. 2 were closed by the
end of the study after endoscopic ablation. Of the remaining 4, 2 were yet to
be closed amongst which one, who had vesicostomy at the age of 3 weeks was lost
to follow-up and was later seen at the age of 6 years in the prospective phase
of the study ; and 2 had died at 2 days post -op. of septicaemia. BLOCKSOM
vesicostomy is a tubeless bladder diversion. It was first performed by BLOCKSOM
in 1956 in a 75 year old man with carcinoma of the urethra (42). The advantages
are that it is tubeless, readily reversible, easy to perform and does not
require any appliance. By decompressing the urinary tract it allows a very ill,
often azotemic and septic child to recover from the long-term effects of
obstruction or severe reflux (42). Definitive reconstructive surgery can be
postponed until the patient's condition is optimal, renal function has improved
or stabilized, infection is finished or until the patient's size is more
appropriate for the particular procedure.
All the patients who underwent cystostomy later underwent
endoscopic ablation. Two cases of catheter ablation were done before the advent
of endoscopic surgery in Cameroon. 1 later underwent endoscopic resection and
the other went into end-stage renal failure and died.
Secondary procedures were performed in only 11 patients. High
diversions were performed in 6 cases - 3 ureterostomies and 3 nephrostomies for
severe bilateral hydronephrosis. All the ureterostomies and nephrostomies were
closed Primary ureteroplasties were done in 4 patients. One patient had
urethrostomy for meatal stenosis following catheter ablation done elsewhere.
Catheter ablation is abandoned in our institutions since the advent of
endoscopic surgery.
Although controversy still exists as to the management of PUV,
the current attitude is a primary valve ablation followed by observation and
vesicostomy reserved for patients in whom valve ablation is not technically
possible or in a child with severe renal failure .(43, 44,31,45). The long-term
outcomes with primary diversion and primary valve ablation are the same, but
performance and reversibility of diversion
72
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
requires more major surgical procedures (31).
IV. OUTCOME OF THE PATIENTS (Table 15)
The overall mortality was 21 %. COULIBALY et al (18) had 15%;
FALL et al (20)14%, LOTTMAN (46) 4% and WARSHAW et al (38) 4.5%. 10 patients
(36%) were lost to follow-up
Causes of deaths: 3 died of septicaemia, 2 post-obstructive
diuresis and 1 of chronic renal failure
* Septicemia:1 patient (2 months old) died 2 days after an
emergent vesicostomy for uraemia, from Pseudomonas septicaemia. l (3 months)
died of klebsiella sepsis and cardiac decompensation and 1 (2 years old) died
of klebsiella pneumonia and Enterobacter cloacae septicaemia one week
after having undergone endoscopic resection and ureteroplasty for bilateral
hydronephrosis.
* Chronic renal failure: The patient who died of chronic renal
failure was 18 years old. He had undergone catheter ablation in the neonatal
period, developed end-stage renal failure at 7 and had a kidney transplantation
(the donor was the mother) in France. Before transplantation he had had a
nephrostomy and several peritoneal dialysis. Back home in Cameroon, he was on
immunosuppressors (Cyclosporine and Azathioprine) developed skin Kaposi
sarcoma, severe lung infection graft rejection and died.
* Post -obstructive diuresis: One patient (9 days old) died 2
days after an emergency BLOCKSOM vesicostomy for uraemia. The other 4 months
old died 2 months after endoscopic resection, in a hospital out of Yaounde. He
had developed gastro-enteritis, and this added to the polyuria he has been
having and inadequate dehydration caused severe dehydration and death.
Post-obstructive diuresis is persistent polyuria following
valve ablation or relief of any obstruction of the urinary (47, 48). This can
provoke a dramatic urinary loss of salt and water and hypotonic urine. Severe
polyuria carries a risk of dehydration, particularly with diarrhoea and
vomiting or high solute feeds (47). There are two major causes: (48)
1) Urea, through its osmotic effect and possibly natriuretic
humoral substances 73
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
which inhibit the reabsorption of NaCl and water in the proximal
and distal tubules.
2) Obstruction per se, possibly through increased pressure within
the renal pelvis
inhibits the reabsorption of fluid and loss of urine
concentration ability. Appropriate but cautious fluid replacement should be
administered to patients with post-obstructive diuresis depending in large, on
what is excreted.
V. RENAL FUNCTION (Tables 6 and 17)
Of the 12 patients we had in the prospective phase, renal
function tests (BUN and creatinine) were done only by 9 (3 did not do because
of financial constraints). BUN is not a good predictor of renal function
because of its variability with factors as dietary proteins, fever, hydration
and liver damage. GFR as calculated from timed urine collection and serum
creatinine is often inaccurate in infants because of problems in collection of
the urine (45). A nadir creatinine valve less than or equal to 0.8 mg /dl by 12
months of age has been described a good predictor of good renal function at the
time of final evaluation (38). Serum creatinine is valid only as it relates to
muscle mass, therefore a blanket endorsement of 0.8 mg/dl at 1 year of age
doesn't take into account the variability in body size (45). So we calculated
the GFR from COCKCROFT's FORMULA considering the weight, age and serum
creatinine. No patient had GFR above 100 ml/min/1.73m2 at diagnosis
and only 2 had GFR above 50 ml/min/1.73m2 at diagnosis. At the end
of the follow-up 6 patients had improved GFR (one above 100 ml/min/1.73
m2, two between 50 and 100 ml/min/1.73 m2 and three below
50 ml/min/1.73m2. Contrarily in 2 patients it dropped, from 46 to 30
in one, and from 61 to 36 ml/min/l.73m2 in the other. In one patient
it remained stable at 15 ml/min/1 .73m2. An important observation is
that despite these low GFR, these children manifested no signs of renal
failure.
In man, the relationship between the duration of obstruction
and the degree of recovery of renal function after release is not known (48).
Return of function depends upon many factors other than the length of
obstruction, such as absence of infection,
74
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
presence of an intrarenal or extrarenal pelvis in the
obstructed kidney, and the degree of pyelolymphatic and pyelovenous blood flow
(48). However the ultimate objective in the management of infants and children
with obstructive uropathies is long-term preservation of renal function
(36).
Chronic renal failure occurs in a significant number of
children with a history of posterior urethral valves (32, 49). Causes include
intrauterine renal dysplasia and hydronephrotic damage, vesico-ureteral reflux,
continued bladder outlet obstruction and vesical dysfunction, (23, 49),
postnatal UTI, hyperfiltration and glomeoulosclerosis. Abnormal bladder
compared to these other factors for causing end-stage renal disease remains
unknown (49). The valve bladder syndrome, involves a poorly compliant,
small capacity bladder leading to upper tract dilatation and renal compromise
that is amendable to improvement via bladder augmentation with intestine
(32).
Although the choice of surgical treatment for patients with
PUV often involves an attempt to stop the course of progressive renal failure,
many are born with severe renal dysplasia that leads to inevitable progressive
renal failure regardless of the primary method of treatment (32). HENNEBERRY
and STEPHENS proposed that renal dysplasia associated with posterior urethral
valves is not secondary to reflux or transmitted high pressures, but rather
results from aberrant caudal budding of the ureter from the mesonephric duct
with subsequent abnormal induction of mesenchyma (37). Renal dysplasia occurs
before the 10th week of gestation before ultrasound can diagnose
(3). The experience of CLOSE C.E. et al (32) suggest that there is a window for
healing in neonates that is limited to the first few months of life with
primary valve ablation.
Ultrasonographic demonstration of corticomedullary junctions
in infancy appears to be a useful, favourable prognostic index in boys with
posterior urethral valves and possibly other obstructive uropathies (50). The
single ultrasonographic parameter showing significant correlation with eventual
renal function was the appearance of the corticomedullary junctions. Additional
ultrasonographic findings, including hydroureteronephrosis, cortical
echogenicity, cortical thickness, bladder wall thickness
75
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
and the degree of posterior urethral dilation, had poor
predictive valve.
VI. GROWTH (Table 17 and NCHS Growth Charts)
We had the weights of the 9 patients assessed for renal
function. Most preoperative heights were not available so we considered only
the pre-operative and post-operative weights. The weights were plotted onto
NCHS ( NATIONAL CENTER FOR HEALTH STATISTICS ) Growth Charts.
Pre-operatively there was growth retardation in 8 patients and 1
had a weight at the 50th percentile.
Analysis showed:
> Between the 25th and 50th percentile : 2 patients >
Between the 10th and 25th percentile : 3 patients > Below the 5th percentile
: 3 patients
At the final evaluation, 5 patients had improvement in their
growth curves and in 4 there was regression in growth.
Analysis showed:
> Above the 95 percentile: 1 patient
> Between the 75th and 90th percentile: 1 patient >
Between the 25th and 50th percentile: 1 patient > At the 25th percentile: 2
patients
> Between the 10th and 25th percentile: 1 patient > At the
10th percentile: 1 patient
> Between the 5th and 10th percentile: 1 patient > Below
the 5th percentile: 1 patient
The mean age at diagnosis of the 5 patients who had an
improved growth was 11.4 months and that of the 4 who had regression of their
growth curve was 42.3 months. KRUEGER R.P et al (15) noted that the follow-up
growth potential was less in those patients presenting at the youngest age and
improved as the age of presentation 76
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
increased. KRUEGER's explanation of his findings was that if
renal impairment occurs in infancy, the growth failure is more pronounced than
is when renal disease occurs later in life. According to YURI REINBERG et al
(51) renal function is the only predictor of body growth.
A possible explanation to our findings could be that patients
in our series had less obstructing valves with onset of the renal impairment
later in life see table 6. We compared the pre-operative GFR of those who had
improved growth with those with regression in growth and it was 26.6
ml/min/l.73m2 and 32.75 ml/min/l.73m2. When we analysed
the GFR in the two groups by the WILCOXON's Rank Sum Test, P was 0.46 so
greater than the 5% level of probability (P > 0.05) indicating that the
difference in GFR in the two groups is statistically non-significant. Our
sample was probably too small to draw meaningful statistical conclusions from
the association body growth and GFR at diagnosis.
According to KRUEGER et al (15) growth failure is more
apparent with regard to linear growth but is also manifested as a failure to
gain weight. It is reported that a GFR of 25 to 30 ml/min/1.73m2 is
the threshold under which growth begins to be stunted, but this figure must be
considered a rough approximation, and some children continue to grow at their
centiles with a lower GFR (52).
Growth retardation is one of the most striking effects of
chronic renal failure in childhood (52). Among the factors that may interfere
with growth in children with renal insufficiency are nephrosis with massive and
permanent proteinuria entailing severe protein depletion, water and electrolyte
disturbances, hypertension, anaemia, renal osteodystrophy, hormonal and
metabolic disturbances and protein -energy malnutrition (52).
VII. INCONTINENCE
Incontinence is a frequent complaint of patients treated for
posterior urethral valves. It was present in 8 cases (29%) in our series. It
occurred in 19% of patients in
77
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
CONNOR and BURBIGE's study (53). According to COCHAT (19) it
is present in 10-30% of cases of PUV and gradually disappears after puberty,
following growth of the prostatic tissue. Formerly it was thought to be due to
surgical trauma on the bladder neck and distention of urethral musculature
bladder dysfunction (53, 9). The loss of urine concentrating ability leading to
large volumes of dilute urine, has been reported in boys with PUV (54). The
combination of polyuria with poor bladder dysfunction or compliance almost
inevitably causes incontinence (10, 54). Therapy includes, clean intermittent
catheterization and anticholinergic medications. Augmentation cystoplasty may
be needed in case of high intravesical pressures despite anticholinergic
therapy (19, 54).
78
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
CONCLUSIONS AND
RECOMMENDATIONS
79
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
Despite our efforts to request parents to bring their children
for follow-up, we were very disappointed with the poor turn out. Of the 22
patients not known to have died at the beginning of the study only 12 came, but
then only 9 were able to do simple renal function tests as BUN and creatinine
not to talk of control ultrasounds and cystourethrograms. The other 3 went away
promising to come back at least with results of BUN and creatinine but they are
yet to return. One patient who had vesicostomy at the age of 3 weeks and was
lost to follow-up turned up after receiving our message. Although he was doing
fine and going to school with the renal function satisfactory (GFR 4
ml/min/1.73m2 at 3 weeks of age to 92 ml/min/1..73m2 at
the age of 6 years) there was already stomal stenosis. The mother promised to
bring him back for closure of the vesicostomy but is yet to come.
The mean number of follow - up visits was 3 (range 0-10) which
is not adequate. It is undoubted that more than 28 patients with posterior
urethral valves were managed in the three hospitals from 1985 to 1997 but poor
keeping of patient records in the archives didn't permit us to have more.
Simple information as address, weights, heights, laboratory and radiological
investigations were lacking in most files.
We thus recommend:
1) That a large scale study be done in Cameroon to determine the
incidence of PUV as well as predictive factors which determine the long term
renal status.
2) That medical records of patients be well kept with all
investigations, growth charts and especially the patients' address The archives
system should be completely renovated.
3) To the obstetricians:
> That the ammiotic fluid be carefully assessed as part of
routine prenatal visits. In case of oligohydramnios, obstructive uropathy
should be suspected and an ultrasound requested so that management should be
started at least soon after birth. While in some series (31) more than 40% of
PUV are diagnosed in the first month of life, 50% of our cases were diagnosed
above 1 year with a mean age at diagnosis of 2.9 years
80
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
4) To the radiologists:
> Any ultrasound in the early second trimester (16 - 18
weeks) should
explore the urinary system to look for signs of obstructive
uropathy.
5) To paediatricians:
> That PUV should not be regarded as a rare entity in
Cameroon. We are convinced that there are many more undiagnosed cases in our
health institutions. As CAMPBELL stated (cited in 35) «Prostatic
urethral valves are not rare, they are just rarely identified».
> that the complications of PUV be well understood and
management known. Three patients died of septicaemia and two of post
-obstructive diuresis . These deaths could have at least been reduced if
appropriate measures were taken promptly.
> Any child with a urinary tract infection or suspected
symptoms should benefit from urine cultures and radiological investigations (at
least ultrasonography) because these could be first manifestations of
obstructive uropathy.
> The urinary stream of children should be clinically
evaluated during routine consultations and any abnormal stream should be
investigated. We were unable to recruit a 32 year old man (because he was out
of town) who had been undergoing hemodialysis for chronic renal failure and was
diagnosed and treated for PUV at the age of 21 years. He has been having mild
symptoms since infancy and these did not draw attention towards PUV.
This is an illustration of mild cases which evolve undiagnosed
to end - stage renal failure and as HENDREN (37) rightly states: «the
picture as usually described is but one end of a spectrum and that
there are many less severe and dramatic cases which escape
recognition».
81
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
BIBLIOGRAPHY
82
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
1 Denes ED, Barthold JS, Gonzalez R. Early prognostic valve of
serum creatinine levels in children with posterior urethral valves. J Urol 1997
Apr; 157(4): 1441-3.
2 Hendren HW. Posterior urethral valves in boys. a broad clinical
spectrum. J Urol 1971 ; 106 : 298 - 307.
3 Audry G, Montagne JP, Brueziere. Le dépistage in-utero
des malformations urinaires. Conduite à tenir. Ann Urol 1992;26(4):
197-201.
4 Atwel JD. Posterior urethral valves in the British Isles. A
Multicenter B.A.P.S. review. J Paed Surg 1983 Feb; 18(1):70-4.
5 Angwafo F, Andze G, Biouele JM, Sosso MA, Edzoa T, Niat G. Les
valves de l'urètre postérieur chez l'enfant. A propos de 22 cas.
J Urol 1995; 101(3) : 132-7.
6 Aaronson IA.Posterior urethral valve masquerading as the prune
belly syndrome. Br J Urol 1983;55: 508-12.
7 Baunin C, Puget C, Gafsi R.Troubles mictionnels
révélateurs de valves de l'urètre postérieur :
aspects radiologiques. Arch Pédiatr 1997; 4(Suppl. 1): 14s-18s.
8 Colodny A.Urethral lesions in infants and children in:
«adult and pediatric urology» 1987
pp 1782 -- 1807, edited by Gillenwater JY, Grayhack JT, Howards
SS, Duckett JW. Year Book Medical Publishers, inc. Chicago, London, Boca Raton;
1st edit, vol 2.
9 De Gennaro M, Mosielo G, Capitanucci ML, Silver M, Capozza N,
Cmone P.
Early detection of bladder dysfunction following posterior
urethral valves ablation. Eur J Pediatr Surg 1996;6:163-5.
10 Dinneen MD, Duffy PG. Posterior urethral valves. Br J Urol
1996; 78: 275-81.
11 Garry S. H. Anomalies of the bladder and urethra.In:
Uropathology. Churchill Livingstone, New York, Edinburgh, London , Melbourne
1989;1st edit. vol 1: pp 235-277.
12 Good CD, Vinnicombe SJ, Minty IL, King AD, Mather SJ, Dicks -
MC. Posterior urethral valves in male infants and new-borns : detection with US
of the urethra before and during voiding. Radiology 1996; 198: 387-91 .
13 Hulbert WC, Duckett JW. Current views on posterior urethral
valves. Pediatr Ann 1988 Jan;17: 1.
14 Karl -Heinz K, Alleman EEJ, Schrôder FH. Major and minor
complications of posterior urethral valves. J Urol 1981; 126: 517-9.
15 Krueger RP, Hardy BE, Churchill BM. Growth in boys with
posterior urethral valves: primary valve resection vs upper tract diversion.
Urol Clin North Am 1980 Jun ;7(2):265-72.
16 Rajab, Freeman NV, Patton M. The frequency of posterior
urethral valves in Oman (abstract) Br J Urol 1996 Jun;77(6): 900-4.
17 Rittenberg MH, Hulbert WC, Snyder, Duckett JW. Protective
factors in posterior urethral valves ( abstract). J Urol 1988 Nov;140(5):
993-6.
83
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
18 Coulibaly B, Dick B, Bankole R, Demoulet C, Gmagne YM ,
Moussa, et al. Les valves de l'urètre postérieur chez le nouveau
- né, le nourrisson et l'enfant : a propos d'une série de 60 cas.
J Urol 1994 ; 100(2) : 87-91.
19 Cochat P, Faraj G Schell, Ulmer S, Parchoux B, Dubois R,
Pouillaude JM, et al. Les valves de
l'urètre postérieur de la période
anténatal a l'age adulte Arch Pediatr 1996;3 :1059-63.
20 Fall I, Ba M, Gueye SM, Ndoye M, Diagne BA, Mensah A, et
al.Valves de l'urètre postérieur chez l'enfant
sénégalais: a propos de quatorze observations. Ann Pédiatr
( Paris) 1992 ; 39(6) : 375 - 80.
21 Yuri Reinberg, Iris De Castano, Ricardo Gonzalez. Prognosis
for patients with prenatally
diagnosed posterior urethral valves. J Urol 1992 Jul ;
148:125-6.
22 Rickham RP, Sopper RT, Stauffer.Urinary disorders. In:
Synopsis of pediatric surgery. Georg Thieme Publishers, Stuttgart 1975; pp:
309-311.
23 Hwang Choi.Valves and prune belly: specific management in the
neonatal period. Current Opinion Urol 1994; 4: 309-12.
24 Churchill BM. Editorial Comments to « Prognostic features
in infants with obstructive uropathy due to posterior valves by Warshaw et
al». J Urol 1985; 133: 240.
25 Bloom W, Fawcett DW. The urinary system. In : A Textbook of
Histology.1975; pp: 766-804 W.B. Saunders Company; Philadelphia - London -
Toronto, 10th Edit.
26 Langman J.The urogenital system. In: Medical Embryology. 4th
edition Williams & Wilkins; Baltimore / London, 1981: pp 234 -2 67.
27 Hanlon-Lundberg KM, Ver MS, Loy G. Posterior urethral valves
in successive generations (Abstract). Am J Perinat 1994 Jan ;11(1): 37-9.
28 Brueziere J, Lasfargues G, Allouch G, Bensman A. Malformations
vesicales. Pathologie de l'ourague. Anomalies du col vésical.
Malformations urethrales. Malformations urinaires complexes : prune belly
syndrome. Encycl Méd Chir, Paris. Pédiatrie, 1981 ; 4083
D30,3.
29 Dewan PA. Type III posterior urethral valves: presentation
and management (letter).
J Pediatr Surg 1996 Jun ;31( 6 ) ;P 867.
30 Dewan PA, Zappala SM, Ransley PG, Duffy PG. Endoscopic
reappraisal of the morphology of congenital obstruction of the posterior
urethral (abstract). Br J Urol 1992 Oct ; 70 (4): 439-44.
31 Smith GHH, Canning DA, Schulman SL, Snyder HM, Duckett JW. The
long - term outcome of posterior urethral valves with primary valve ablation
and observation. J Urol 1996 May ; 155:1730-4.
32 Close Clare E, Cark Michael C, Burns Mark W, Mitchel Michael
E. Lower urinary tract changes alter early valve ablation in neonates and
infants: is early diversion warranted?
J Urol 1997 Mar; 157 :984 -8. 84
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
33 Holmdahl G, Sillen U, Hanson E, Hermansson G,
Hjälmâs. Bladder dysfunction in boys with posterior urethral valves
before and after puberty. J Urol 1996 Feb ;155 : 694-8.
34 Nnomzo'o E. Uropathies Malformatives Congénitales De
l'enfant Observée A YaoundéThèse CUSS 1990,
Yaounde, Cameroon.
35 Tanagho EA, Smith DR. Urology. In: Current Surgical
Diagnosis and Treatment. Edited by J. Englebert Dunphy and Lawrence W. Way.
Lange Medical Publications, Los Altos, Carlifornia 3rd Edition 1977; pp:
817-866.
36 Warshaw BL, Hymes LC, Woodard JR. Long - term outcome of
patients with obstructive uropathy.Pediatr Clin North Am 1982
Aug;29(4):815-26.
37 Henneberry MO, Stephens FD. Renal hypoplasia and dysplasia in
infants with posterior urethral valves. J Urol 1980 ;123: 912-5.
38 Warshaw BL, Hymes LC, Woodard JR.Prognostic features in
infants with obstructive uropathy due to posterior urethral valves. J Urol
1995;133:240-3.
39 Kupferman JC, Stewart CL, Kaskel FJ, Fine RN. Posterior
urethral valves in patients with down syndrome ( abstract). Pediatr Nephrol
1996 Apr ;10(2): 143-6.
40 Walker RD, Richard GA, Bueschen AJ, Retik AB. Pathophysiology
and recoverability of function and structure in obstructed kidneys. Urol Clin
North Am 1980 Jun; l7(2) :291-310.
41 Moscovici J. Troubles mictionnels révélateurs
d'une valve de l'urètre postérieur: aspect urodynamique. Arch
Pédiatr 1997; 4 (1): 19s-22s.
42 Hurwitz RS, Ehrlich RM.Complications of cutaneous vesicostomy
in children.
Urol Clinic North Am 1983: 10(3):503-8.
43 Kim YH , Horowitz M, Combs A, Nitti VW, Libretti D,
Glassberg KI. Comparative urodynamic findings after primary valve ablation,
vesicostomy or proximal diversion. J Urol 1996 Aug; 156: 673 --6.
44 Myers DA, Walker RD. Prevention of urethral structures in the
management of posterior urethral valves. J Urol 1981; 126: 655-7.
45 Walker R.D., Padron Manuel. The management of posterior
urethral valves by initial vesicostomy and delayed valve ablation. J Urol 1990;
144: 1212-4.
46 Lottman H, Melin Y, Cendron J.Valves de l'urètre
postérieur. Chir Pediatr 1986 ; 27:15 -26.
47 Dinneen MD, Duffy PG, Barratt TM, Ransley PG. Persistent
polyuria after posterior urethral valves. Br J Urol 1995 ;75: 236-40.
48 Saulo Klahr, Buerkert J, Morrison A. Urinary tract
obstruction. In: «The_Kidneys». Edited by Brenner and Rector,
Published by Ardmore Medical Books W.B. Saunders Company, 1986; PP:1443-90.
49 Laurent Salomon, Fontaine E, Gagnadoux M-F, Broyer M, Beurton
D. Posterior urethral valves:
85
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
long-term renal function consequences after transplantation. J
Urol 1997 Mar;157 : 992-5.
50 Hulbert WC, Rosenberg HK, Cartwright , Duckett JW, Snyder
HM. The predictive value of ultrasonography in evaluation of infants with
posterior urethral valves. J Urol 1992 Jul; 148: 122-4.
51 Reinberg Y, De Castano I, Gonzalez R, Duckett JW. Influence
of initial therapy on progression of renal failure and body growth in children
with posterior urethral valves. J Urol 1992 148: 532 -3.
52 Broyer M. Growth in children with renal insufficiency. Pediatr
1982 Aug. Ped Clin North Am 1982 Aug; 29(4):991-1003.
53 Connor JP, Burbige KA. Long term urinary continence and renal
function in neonates with posterior urethral valves. J Urol 1990 Nov; 144(5):
1209-11.
54 Bouche PM, Lefort G, Daoud S.Neonatal urinary ascitis caused
by posterior urethral valves. a propos of 2 cases (abstract). Chirurg
Pédiatr 1987; 28 (1): 52-5.
55 Burstein JD, Firlit CF. Complications of cutaneous
ureterostomy and other cutaneous diversion.
Urol 1983 Aug; Clin North Am 1983 Aug: 10 (3):433-43.
56 Charbit L, Cukier J, Boiteux F. Relationships between
posterior urethral valves, vesico - renal reflux and renal dysplasia
(Abstract). Acta Urologica Belgica 1990; 58 (1): 73 - 7.
57 Choudhury SR, Mitra SK, John P. Parietal wall urinary
extravasation and abdominal wall hernia secondary to posterior urethral valves
in a neonate. Br J Urol 1995; 76: 800-12.
58 Churchill BM, Krueger RP, Fleisher MH, Hardy BE. Complications
of posterior urethral valve surgery and their prevention. Urol Clin North 1983
Aug; 10 (3):519-30.
59 Davody AP, Amaro JW, Cukier 3. Posterior urethral valves in
new-borns and infants. treatment and clinical course ( abstract). Prog Urol
1992 Oct ; 2 (5): 901-7.
60 Dell'agnola CA , Tomaselli V , Ferrazi F, Kustermann A,
Nicolini U. Perinatal ultrasound
monitoring: early detection and treatment of congenital uropathy.
Br J Urol 1983 ;55: 469-72.
61 Ditchfield M R, Grattan - Smith, John D, De Campo, John M.
Voiding cystourethrography in boys : does the presence of the catheter obscure
the diagnosis of posterior urethral valves? Am J Roentg 1995; 164:1233-5.
62 Gordon I, Ranslfy PG, Hubbard CS. 99m Tc DPTA scintigraphy
compared with intravenous urography in the follow-up of posterior urethral
valves. Br J Urol 1987 Nov; 60 (5): 447-9.
63 Guys JM., Meyrat B, Simfoni - Alias J, Coquft M., Monthort G.
Les troubles mictionels persistent après traitement d'un valve de
1'urètre postérieur : incidence et sémiologie. Arch
Pédiatr 1997; 4 (1): 27s-30s.
64 Hoebeke P, Van Laeke E, Raes A, Vande Walle J. Troubles
mictionnels révélateurs d'une valve de l'urètre
postérieur: aspects cliniques. Arch Pédiatr 1997; 4 (1):
10s-13s.
65 Hutton KA, Thomas DF, Arthur RJ, Irvinf HC, Smith SE.
Prenatally detected posterior urethral 86
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
valves: is gestational age at detection a predictor of outcome? J
Urol 1994 Aug; 152 : 698-701.
66 Kaefer Martin, Barnewolt Carol, Retik Alain B, Craig A
Peters.The sonographic diagnosis of infravesical obstruction in children:
evaluation of bladder wall thickness indexed to bladder filling. J Urol 1997
Mar ;157: 989-91.
67 Lepinard Berbesson C. Echographie et malformations urinaires
foetales.Ann Urol 1996; 20(4):
225-32.
68 Melekos MD, Asbach HW, Giannoulis S, Perimen's P, Barbalias
G.Aspects concerning posterior urethral valves (abstract). Intern Urol Nephrol
1989; 21 (1): 57-62.
69 Mildenberger H, Habenicht R, Zimmermann H. Infants with
posterior urethral valves : a retrospective study and consequences for therapy
( abstract). Prog Pediatr Surg
1989 ;23 :104-12.
70 Monfory G, Morisson - Lacombe G, Bensoussan A, Carcassonne M.
Les valves de l'urètre postérieur chez le garçon. Ann Chir
Infant 1976 ; 17: 15 - 33.
71 Montagnino B. Posterior urethral valves: pathophysiology and
clinical implications. ANNA J 1994 ;30 Feb ;21(1): 26-30.
72 Mouriquand PDE.Valves de l'urètre postérieur:
facteurs déterminant les résultats à long terme. Arch
Pédiatr 1997; 4(1) : 31s-36s.
73 Nakayama DK, Harrison MR, De Lorimier AA. Prognosis of
posterior urethral valves presenting at birth (abstract). J Pediatr Surg 1986
Jan ;21(1): 43-5.
74 Ng Jacob WT , Chan Andrew YT, Kong CK., Wong MK.Posterior
urethral valves presenting as acute epididymo-orchitis : a case report and
follow - up study. Aust N Z J Surg, 1996; 66:129-30.
75 Parkhouse HF, Baratt TM, Dillon MJ, Duffy PG; Fay J, Ransley
PG et al.Long-term outcome of boys with posterior urethral valves (abstract).
Br J Urol 1988 Jul; 62(1): 59-62.
76 Peters CA , Bolkier M, Balier SB, Hendren WH, Colodny AH,
Mandel J, et al. The urodynarnic consequences of posterior urethral valves
(abstract). Br J Urol 1938 Jul ; 62(1): 59-62 .
77 Pompino HJ, Bodecker RH, Trammer UA.Urethral valves during the
first year of life:a retrospective , multicenter studyEur. J Pediatr Surg 1995;
5: 3-8.
78 Prem Puri, Rajendra Kumar.Endoscopic correction of
vesicoureteral reflux secondary to posterior urethral valves. J Urol 1996 Aug ;
156: 680 - 2.
79 Sarkis P, Robert M, Lopez C, Veyrac C, Gutter J, Averous M.
Obstructive anuria following fulguration of posterior urethral valves and foley
catheter drainage of the bladder. Br J Urol 1995; 76: 664-665.
80 Saul P. Greenfwld.Posterior urethral valves ; new concepts
(editorial). J Urol 1997 Mar;
157:996-7.
81 Sauvage P.Les aspects endoscopiques des valves de
l'urètre postérieur. Arch Pediatr 1997 ; 4 (1) :
23s-26s.
82 Tejani A, Butt K, Glassberg K, Price A, Gurumurthy K.
Predictors of eventual end stage renal 87
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
disease in children with posterior urethral valves. J Urol 1986
Oct ;136(4): 857-60. 83 Thomalla JV, Mitchel ME, Garett RA. Posterior urethral
valves in siblings.
Urology 1989 Apr; 33(4): 291-4.
88
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|

APPENDIX
89
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
YAOUNDE GENERAL HOSPITAL P.O. BOX 5408
20-11-22 20-14-59 20-16-78 Dear Madame / Sir,
You are kindly requested to bring your child
who had Urinary tract problems and was followed -up by Dr
ANGWAFO, for a control check -up.
This control examination is free -of - charge and even urgent
because these children might develop long - term complications.
Please bring along the medical file (x-rays and laboratory
investigations).
You should contact Dr CHIABI Andreas (Paediatric resident) in
the Paediatric service of the Yaounde General Hospital if you come before March
1997; if later contact me in the Pediatric service of the Yaounde Central
Hospital Pavillon Jeanne Irene BIYA.
Thanks for your co-operation.
Dr ANGWAFO
P.O. Dr CHIABI Andreas
90
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
QUESTIONNAIRE ON POSTERIOR URETHRAL VALVES IN YAOUNDE 1.
IDENTITY
|
Name: Hospital File N°:
Date of Birth Age Residence:
Tribe:
Address:
|
II. PAST HISTORY FAMILY HISTORY
UTI Yes [ ] No [ ] Renal Disease Yes [ ] No [
]
Number Malformations Yes [ ] No [ ]
Germ Crytochidism Yes [ ] No [ ]
Hypospadias Yes [ ] No [ ]
Others:
|
|
III. PRESENTING COMPLAINTS
Fever
Anorexia
Failure of thrive
Nausea
Vomiting
Diarrhoea
Dehydration
Respiratory distress
Pollakiuria
Nocturia
Dysuria
Hermatuna
Dribbling
Urine retention
Incontinence
Enuresis
Chronic renal failure
Others:
Age of 1st consultation: Age of diagnosis:
91
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
IV.
PHYSICAL FINDINGS
Weight: P50: Height:
|
|
P50:
|
BSA:
|
|
No [ 1
|
Ext. Urogenital Malformations: Yes [ 1
Type :
|
|
|
|
Abdominal Mass: Yes [ 1
Location:
|
|
No [ 1l
|
Others:
|
|
|
V. INVESTIGATIONS
|
|
|
|
|
CBC: WBC: PN:
|
|
PL:
|
|
RBC :
|
|
|
|
FIB: MCV:
|
|
Urine Culture: Positive: [ ] Negative: [
|
]
|
|
Germ :
|
|
WBC: RBC:
|
|
BUN: Creatinine:
|
|
|
Creatinine Clearance:
|
|
|
|
K+ Na+
|
|
CL
|
|
|
|
|
Ultra Sound: Antenatal: Yes [ ] No [ ]
|
|
|
|
Normal [ ] Abnormal [ ]
Postnatal:
|
Precise:
|
|
|
YES
[ ]
[ ]
[ ]
[ ]
|
NO
[ ]
[ ]
[ ]
[ ]
|
|
Amenorhoea:
-Uretero Hydronephrosis: Trabeculated bladder: Dilated post.
urethral:
Renal cortex:
|
|
Renal size:
|
|
|
|
Others :
|
|
|
|
Voiding Cystourethrogram
|
YES
[ ]
[ ]
[ ]
[ ]
|
NO
[ ]
[ ]
[ ]
[ ]
|
|
VUR
Trabeculated bladder Dilated post. urethra Presence of
valve
Others:
|
|
|
|
|
IVP --Normal Uretero Hydronephrosis Yes [ ] No [
]
|
|
|
|
Stage:
|
|
Symetry: Yes [ ] No [ ]
|
|
|
|
Late secretion: Yes .[ ] No [ ]
Others:
|
|
|
|
|
|
92
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
VI. TREAMENT
VII.

Medical
Surgical
FOLLOW-UP
Stream: Normal [ ] Abnormal [ ]
Renal function: Stable [ ] Improved [ ]
Deteriorated [ ]
Ultrasound:
Voiding CystoUrethrogam:
VUR Yes [ ] No [ ]
Urethral Structure Yes [ ] No [ ]
Diverticulum: Bladder [ ] Urethral [ ] None [ ]
IVP: Normal Uretero Hydronephrosis Yes [ ] No [ ]
Stage Yes [ ] No [ ]
Symetry Yes [ ] No [ ]
Late secretion Yes [ ] No [ ]
Incontinence Yes [ ] No [ ]
Lost of follow-up Yes [ ] No [ ]
Dead Yes [ ] No [ ]
If yes cause:
|
|
Others:
VIII. CONCLUSION:
93
|
|
|
|
POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
|
VARIABLES
DATES
94

POSTERIOR URETHRAL VALVES IN CHILDREN: A review of 28
cases in Yaounde
| 


