
 |
Contrainte Psycho-Physiques et Electrophysiologiques sur le codage de la stimulation électrique chez les sujets porteurs d'un implant cochléaire( Télécharger le fichier original )par Stéphane GALLEGO Université Lyon I - Doctorat 1999 |
- Article 22 :MISTMATCH NEGATIVITY : A TOOL FOR THE ASSESSMENT OF
STIMULI DISCRIMINATION IN
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Subject |
Age |
Etiology |
Total deaf duration (yr) |
Implantation |
Electrode pairs tested |
|
JL |
59 |
otosclerosis |
4 |
8 |
13:33, 12:35, 10:31, 8:26 |
|
AN |
40 |
meningitis |
6 |
4 |
13:64, 12:66, 10:68, 8:68 |
|
HB |
69 |
chronic otitis |
2 |
21 |
13:22, 12:26, 10:29, 8:36 |
|
SO |
71 |
otosclerosis |
1 |
8 |
13:31,10:44, 8:38 |
|
MI |
55 |
sudden deafness |
1 |
6 |
13:16, 12:18, 10:18, 8:16 |
|
AZ |
53 |
sudden, meningitis |
7 |
12 |
13::14, 12:14, 10:16, 8:18 |
|
-SP |
65 |
sudden deafness |
13 |
28 |
13:24, 12:28 |
|
AC |
59 |
progressive deafness |
3 |
14 |
13:23, 12:25, 10:24, 8:27 |
Table II- Characteristics and evaluation criterion of speech tests.
Test Characteristics level of evaluation
VCV 16 consonants, 3 times each consonant /48
Lafon 17 monosyllabic words of three phonemes /51
phonemes
words 75 familiar monosyllabic words words /75
sentences 35 sentences, 119 key words key words /119
Table III- Speech test performance for the six subjects included in the statistical analysis.
|
Subject |
VCV |
Words |
Sentences |
Lafon IC |
||
|
ac |
42 |
57 |
80 |
82 |
||
|
an |
27 |
20 |
21 |
50 |
||
|
az |
48 |
45 |
58 |
63 |
||
|
hb |
65 |
41 |
81 |
75 |
||
|
il |
46 |
49 |
84 |
45 |
||
|
mi |
42 |
11 |
21 |
59 |
||
|
S cl) p 15. â |
||||||
0 100 200 300
Time (ms)
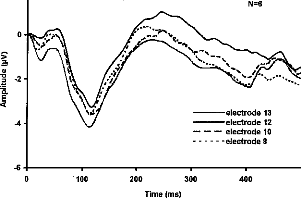
Figure 1-a
0
100
400
200 300
Time (ms)
2
electrode 13 electrode 12
-- -- -- electrode 10 electrode 8
N=6
Figure 1-b
|
pair 1 pair 2 pair 3
Electrode pairs
Figure 2a
|
standard |
pair 1 pair 2 pair 3
Electrode pairs
Figure 2b
4
2
·
·
·
n
·
·n
-4-
·** * * * ** * * * * * *
n standard
· devient
|
hO |
hO 00
|
e b' |
Time intervals (ms)
Figure 3
0
100
200
300
2-
li
standard : electrode 13 deviant : electrode 12 difference : deviant-standard
Time (ms)
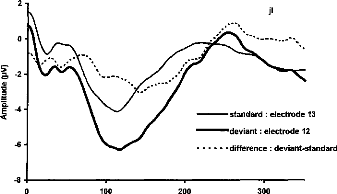
Figure 4-a

.--
...
r \
/ ...
i .p.egrfe.*1
r .s.
·
·
·
i ,
·e.
· IN
i ...%
I ;
· :114.: `
*
·
0
·
·
1,..;
deviant 12 - standard 13 -- -- -- deviant 10 - standard 13 deviant 8 - standard 13
0 100 200 300
Time (ms)
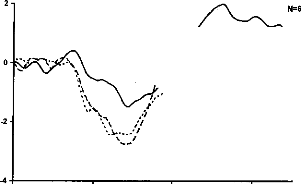
Figure 4-b
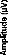
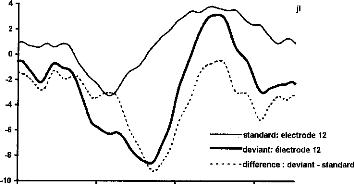
-standard: electrode 12 deviant: électrode 12 difference : deviant - standard
0 100 200 300
Time (ms)
Figure 5-a
4
deviant 12 - standard 12 -- -- -- deviant 10 - standard 10
deviant 8 - standard 8
|
2 |
I
/ % ` e -.5
..
· N
f"
·
·
·
·
· ,...,
%...'"«....
·r..,
i .."0 '
....s s. ''. 1. _.. /--«y
I . . ,.. .
.-------,s\ I ,' ...
·
·...
. ` / .....
. \
.. .".
·... / :
N=6
.. ...... .
|
100 200 300 Time (ms) |
||
|
0 |
||
Figure 5-b
Legend of figures
Figure 1- a- Averaged response of the standard stimulus for a representative subject. The classic PI-NI - P2 wave is obvions, with N1 at about 100 ms. b- grand mean averaged responses from stimulation of electrode 13, 12, 10 and 8 as standard stimuli.
Figure 2- Mean P1N1 amplitude (a) and mean N1P2 amplitude (b) for standard and deviant stimuli in respect to electrode pairs.
Figure 3- Mean ERP values for standard and deviant stimuli by 50 ms periods. The stars indicate the signification level of the comparison for each interval. *: p<0.05, **: p<0.01, ***: p<0.001.
Figure 4-a- Averaged responses of the stimulation of electrode 12 as standard stimulus, as deviant stimulus, and difference wave for a representative subject. The MMN deflection is obvions in the response of the deviant stimulus, overlapping the N1-P2 wave. b- Grand mean MMN intra-session difference wave (N=6) for the three pairs of electrodes.
Figure 5-a- Averaged responses of the standard stimulus (stimulation of electrode 12), of the deviant stimulus (stimulation of electrode 12), and difference wave for a representative subject. A negativity is obvions in this difference wave. b- Mean MMN nitra-session difference wave (N=6) for the three pairs of electrodes.
Références
Abbas PJ, Brown CJ. Electrically evoked auditory brainstem response: Growth of the response with current level. Hear Res 1991a 51:123-137
Abbas PJ, Brown CJ. Electrically evoked auditory brainstem response: Refractory properties and strength duration fonctions. Hear Res1991b 51:138-148.
Abbas PJ, Brown CJ. Electrically evoked brainstem potentials in cochlear implant patients with multi-electrode stimulation. Hear Res 1988 36:153-162
Allen JB, Hall JL, Jeng PS. Loudness grwth in 1/2 octave bands (LGOB) a procedure for the assessment of loudness. J Acous Soc Am 1990, 88 745-753
Beliaeff M, Dubus P, Leveau JM, Repetto JC, Vincent P. Sound processing and stimulation coding of Digisonic DX10 15-channel cochlear implant. Hochmair IN, ed. Advances in cochlear implant. Innsbruck: Verlag. 1994 198-203
Berger-Vachon C., Gallégo S., A. Morgon, Truy E.. Analytical Importance of the coding features for the discrimination of wovels in the cochlear implant signal. An Otol Rhinol Laryngol (1995), sup.166,104,9,2, 351353
Berger-Vachon C., Gallégo S., J.C. Bera, E. Arnoux, C. Kissac. A model of vowel representation using a cochlear implant. Proc. Inter. AMSE Symposium. Regio Calabria, (Italie), Sept 11-13 1997
Bertrand O, Perrin O, Pernier J. Evidence for a tonotopic organization of the auditory cortex observed with auditory evoked potential. Acta Otolaryngol (Sotckh) 1991, 491 116-123
Blamey PJ, Dooley GJ, Parisi ESP, Clark GM. Pitch comparison of acoustically and electrically evoked auditory sensation. Hear Res, 1996, 99 139-150
Bregman AS, Levitan R, Liao C. Fusion of auditory components. Effects of frequency of amplitude modulation. Perception and Psychophysics, 1990, 47, 68-73
Brightwell A, Rothera M, Conway M, Graham J. Evaluation of status of the auditory nerve: psychophysical test and
ABR. Eds RA Schindler & MM Merzenich Cochlear Implants Raven Press New York 1985 343-9
Brimacombe JA, Eisenberg LS. Tone decay in subjects with the single channel cochlear implant. Audiology 1984,
23 321-332
Brown CJ, Hughes ML, Lopez SM, Abbas PJ. Relationship between EABR thresholds and levels used to program the Clarion speech processor. Ann Otol Rhinol Laryngol, 1999, 108 50-57
Brown JB, Abbas PJ, Fryauf-Bertschy H, Kelsay D, Gantz BJ. Intraoperative and Postoperative Electrically Evoked Auditory Brain Stem Responses in Nucleus Cochlear Implant Users: Implications for the Fitting Process. Ear Hear 1994 15:168-176
Busby PA, Clark GM. Electrode discrimination by early-deafened cochlear implant patients. Audiology 1996 35:8- 22
Calliope. La parole et son traitement automatique. Ed Masson, 1989
Cazal Y, Pelizzone M, Kasper A, Montandon M. Indication of a relation between speech perception and temporal resolution for cochlear implantees. Ann Otol Rhinol Laryngol 1991 100:893-895
Cazal Y, Pelizzone M, Saudan O, Boex C. Amplitude modulation transfer fonctions associated with speech recognition for Ineraid cochlear implantees. Hochmair IN, ed. Advances in cochlear implant. Innsbruck: Verlag. 1994 321-325
Clark G, Shepherd R, Franz B, Dowell R, Tong Y et al. The histopathology of the human temporal bone and auditory central nervous system following cochlear implantation in patient. Acta Otolaryngol (Stockh) 1988 (suppl 448):165
Clark GM, Tong YC, Martin LFA. A multiple-charnel cochlear implant: an evaluation using closed-set spondaic words. J Laryngol 1981 95:461-464
Cohen LT, Busby PA, Whitford LA, Clark GM. Cochlear implant place psychophysics. Audiol Neurootol 1996, 1 265-277
de Sauvage RC, da Costa DL, Erre JP, Aran JM. Electrical and physiological changes during short-terni and chronic electrical stimulation of the normal cochlea. Hea Res 1997, 110 119-134
Deguine O. Implants cochléaires. Acquisition et controverses. Thèse de médecine, 1990
Dobie RA, Kimm J Brainstem responses to electrical stimulation of the cochlea. Arch Otolaryngol 1980, 106 573577
Don M, Eggermont JJ. Analyses of click-evoked brainstem potentials in man using high-pass noise masking. J Acoust Soc Am 1978 63(4):1084-1098
Donchin E., Ritter W., & McCallum C.W. (1978). Cognitive Psychophysiology : the endogenous components of the ERP. In E. Callaway, P.
Dorman MF, Loizou PC, Fitzke J, Tu Z. The recognition of sentences in noise by normal-hearing listeners using
simulations of cochlear implant signal processors with 6-20
channels. J Ascous Soc Am 1998, 104 3583-3585
Dorman MF, Smith M, Smith L,
Parkin JL. The pitch of electrically presented sinusoids. J Acous Soc Am
1994
95(3): 1677-1679
Doumèche P. L'accès au lexique chez le sujet porteur d'un implant cochléaire. Mémoire d'orthophonie, Paris, 1997 Dynes SBC, Delgutte B. Phase-locking of auditory-nerve discharges to sinusoïdal electrical stimulation of the cochlea. Hear Res 1992, 58 79-90
Eddington DK. Speech discrimination in deaf subjects with cochlear implants. J Acoust Soc Ain 1980 51:885-891 Eggermont JJ. Peripheral auditory adaptation and fatigue : a model oriented review. Hear Res 1985, 18 57-71
Fifer RC, Novak MA. Myogenic Influences on the Electrical Auditory Brainstem Response (EABR) in Humans.
Laryngoscope 1990 100:1180-1184
Fitzgerald P. and Picton T.W. (1983) Event-related potentials recorded during the discrimination of improbable stimuli. Biological Psychology, 17, 241-276.
Friesen LM, Shannon RV, Slattery WH. Speech recognition in noise as fonction of the number of electrodes used in the speak, SAS and CIS speech processors. Confrence on Implantable Auditory Prostheses, Asilomar, August 29 -- September 3, 1999.
Frijns JHM, Snoo SL, ten Kate JH. Spatial selectivity in a rotationally symmetric model of the electrically stimulated cochlea. Hear Res 1996, 95 33-48
Fu QJ, Shannon RV. Effects of amplitude nonlinearity on phoneme recognition by cochlear implant users and normal-hearing listeners. J Ascous Soc Am 1998, 104 2570-2577
Gallégo S. Préservation de l'enveloppe temporelle pour la compression du signal de parole. IVe C F Audiologie 7-8 décembre 1998, Clermont-Ferrand. Sous- presse
Gallégo S. Utilisation de la psycho-acoustique pour le réglage de l'implant cochléaire Digisonic. Conférencié invité au IVe C F Audiologie 7-8 décembre 1998, Clermont-Ferrand. Sous-presse
Gallégo S., B Frachet, Truy E., L Collet. Modification des seuils en fonction des caractéristiques du patient implanté cochléaire. IVe C F Audiologie 7-8 décembre 1998, Clermont-Ferrand. Sous-presse
Gallégo S., B. Frachet, Truy E., Collet L.. Cochlear implant performance and electrically auditory brainstem response characteristics. Electroencephalography & clin neurophysiol. (1998), 108:521-525
Gallégo S., Ba Lê Luu, Berger-Vachon C.. Modelling of the electrical stimulation delivered by the Digisonic Multichannel cochlear implant. AMSE (1998), 39, 1, 39-54
Gallégo S., C. Micheyl, Berger-Vachon C., Truy E., A. Morgon, Collet L.. Ipsilateral ABR with cochlear implant. Acta Oto-Laryngologica. (Stockholm) (1996), 116, 228-233
Gallégo S., C. Micheyl. Intensity discrimination and auditory brainstem responses in cochlear implant and normalhearing subjects. Behaviorial Neuroscience (1998), 112, 4, 793-799
Gallégo S., Collet L., Berger-Vachon C.. A model of electrical stimulation delivered by the Digisonic cochlear implant. World Congress on Medical Physics and Biomedical Engineering, Nice, 14-19 september, 1997. J Int Federation for Medical & Biological Engineering, 35 (suppl), 1, 300
Gallégo S., Collet L., Berger-Vachon C.. Electrically auditory brainstem responses (EABR): contribution of a filter adapted to the auditory system. World Congress on Medical Physics and Biomedical Engineering, Nice, 14-19 september, 1997. J Int Federation for Medical & Biological Engineering, 35 (suppl), 1, 304
Gallégo S., Collet L.. Les méthodes objectives: Potentiels evoqués auditifs électriques précoces pré et post implantation, test d'intégrité. Dossier Entendre n° 7: La réhabilitation des surdités profondes par l'implant cochléaire, 33-40.
Gallégo S., E. Perrin, Berger-Vachon C., Truy E.. Recognition of vowels by cochlear implant using a Fuzzy logic. Proc. Inter. AMSE Symposium `Fuzzy Systems & Neural Networks'. Lyon (France), July 4-6 1994. (AMSE Press) 103-116
Gallégo S., Garnier S., Berger-Vachon C.. Traitement du signal de parole pour sourds-profonds: L'implant cochléaire. revue d'électricité et d'électronique (1997), 8, 50-53
Gallégo S., Garnier S., C. Micheyl, Truy E., A. Morgon, Collet L.. Loudness growth functions and EABR characteristics in Digisonic cochlear implant. Acta Otolaryngol (Stocldi) (1999)
Gallégo S., J. Durrant, Collet L., Berger-Vachon C.. Numeric time-variant filters adapted to the recording of electrically auditory brainstem responses (E-ABR). soumis
Gallégo S., J. Durrant, Truy E., Berger-Vachon C., Collet L.. Effect of stimulation intensity and intracochlear site on electrical auditory brainstem responses in human using a multichannel cochlear implant with a variable duration pulse. soumis
Gallégo S., M. Beliaeff, B. Frachet, M. Ouayoun, Berger-Vachon C., Collet L.. Long-term change in threshold and comfort levels and dynamics in Digisonic cochlear implant bearers. soumis
Gallégo S., M. Beliaeff, Collet L.. EABR with Digisonic cochlear implant: an indispensable objective method for fitting children. Digisonic News, number 2, June 1996
Gallégo S., M. Beliaeff. The long-terra evolution of cochlear implant thresholds. Digisonic News, number 4
Gallégo S., Truy E., A. Morgon, Berger-Vachon C., Collet L.. Les potentiels évoqués auditifs électriques précoces
(PEAEP) sur implant cochléaire: Caractérisation et applications cliniques. 4ème congrès français d'Acoustique.
14-18 avril 1997, Marseille, Vol 1, 463-466
Gallégo S., Truy E., A. Morgon, Collet L.. EABRS and surface potentials with a transcutaneous multielectrode cochlear implant. Acta Oto-Laryngologica. (Stockholm) (1997), 117(2), 164-168
Gallégo S., Truy E., Berger-Vachon C., Collet L.. Electrically auditory brainstem responses in cochlear implant assessment: possibility and interest. soumis
Gallégo S., Truy E., Berger-Vachon C., Collet L.. Electrically auditory brainstem responses in cochlear implant as évoqués auditifs électriques précoces (PEAEP) sur implant cochléaire: Caractérisation et applications cliniques. 4ème congrès français d'Acoustique. 14-18 avril 1997, Marseille, Vol 1, 463-466
Gallégo S., Truy E., Berger-Vachon C., Collet L.. Electrically auditory brainstem responses in cochlear implant assessment: possibility and interest. soumis
Garnier S., Gallégo S., Berger-Vachon C., A. Morgon, Collet L.. Application des techniques de codage de l'implant cochléaire à la prothèse auditive conventionnelle. 4ème congrès français d'Acoustique. 14-18 avril 1997, Marseille, Vol 1, 551-554
Garnier S., Gallégo S., Collet L.. Simulation acoustique du message delivré par l'implant cochléaire digisonic de MXM. Cahier de l'audition, 10, 6, 22-24
Garnier S., Gallégo S., V. Ziempfer, Berger-Vachon C.. Simulaton of an hearing aid based on cochlear implant coding. AMSE (1997), 37,1-2, 37-44
Gelis C. Bases techniques et principes d'application de la prothèse auditive. Ed Sauramps. 1993
Gorga MP, Kaminski JR, Beauchaine KA, Jesteadt W. Auditory brainstem responses to tone bursts in normally hearing subjects. J Speech Hear Res 1988 31:87-97
Groen PAP, Makhdoum M, van den Brink, Stollman MHP, Snik AFM, van den Broek. The relation between electric auditory brainstem and cognitive responses and speech perception in cochlear implant users. Acta otolaryngol (Stockh) 1996, 116 786-790
Groenen P, Snik A, van den Broek P. Electrically evoked auditory middle latency responses versus perception abilities in cochlear implant users. Audiology 1997, 36 83-97
Grônfors T. Novel methods of syntactic pattern recognition for peak detection of auditory brainstem responses. Thèse de physique. Kuopio, 1994
Gyo K, Yanagihara N. Electrically and acoustically evoked brainstem responses in guinea pig. Acta Otolaryngol (Stockh) 1980 90:25-31.
Hall RD. Estimation of surviving spiral ganglion cells in the deaf rat using the electrically evoked auditory brainstem
response. Hear Res 1990 of an hearing aid based on cochlear implant coding. AMSE (1997), 37,1-2, 37-44 Hellman R, Miskiewicz A, Scharf B. Loudness adaptation and excitation patterns : effects of frequency and level. J
Acous Soc Am 1997, 101 2176-2185
Herman B, Thornton A. Electrically evoked auditory brainstem responses in cochlear implanted subjects. IInd International Cochlear implant symposium, Iowa city 1992
Hinojosa R, Seligsohn R, Lerner S. Ganglion cell counts in the cochleae of patients with normal audiograms. Acta Otolaryngol (Stockh) 1985 99:8-13
Honert van den C, Stypulkowski PH. Characterization of the electrically evoked auditory brainstem response (ABR) in cats and humans. Hear Res 1986 21:109-126
Honert van den C, Stypulkowski PH. Physiological properties of the electrically stimulated auditory nerve.II. Single fiber recording. Hear Res 1984 14:225-243
House WF, Berliner K, Crary W, Graham M, Luckey R, Norton N, Selters W, Tobin H, Urban J, Wexler M. Cochlear implants. An Otol Rhinol Laryngol 1976 85(suppl 27):1-93
Jewett DL, Williston JS. Auditory evoked far-fields averaged from the scalp of humans. Brain 1971 94:681-96 Kasper A, Pelizzone M, Montandon P. Electrically Evoked Auditory Brainstem Responses in Cochlear Implant Patients. ORL 1992 54:285-294
Kiang NYS et al. Discharge patterns of single fibers in cat's auditory nerve. MIT Res monograph No 35. MIT press, Cambridge, MA, 1965
Kiang NYS, Moxon EC. Physiological considerations in artificial stimulation of the inner ear. Ann Otol Rhinol Laryngol 1972 81:714-731
Kiang NYS. Stimulus coding in the auditory nerve and cochlear nucleus. Acta Oto Laryngol 1965 59:186-200 Kohlrausch A. Comment on 'Temporal modulation transfer functions in patients with cochlear imlants'. J Acous Soc Am, 1993, 93, 1649-1650
Killian MJP, Klis SFL, Smoorenburg GF. Adaptation in the compound action potential response of the guinea pig VIIIth nerve to electrical stimulation. Hear Res 1994, 81 66-82
Kraus N., McGee T., Carrel T., King C., Tremblay K., and Nicol T. (1995) Central auditory system plasticity associated with speech discrimination training. Journal of Cognitive Neuroscience, 7, 1, 25-32.
Kraus N., McGee T., Micco A., Sharma A., Carrel T., and Nicol T. (1993a) Mismatch negativity in school-age children to speech stimuli that are just perceptibly different. Electroencephalography and Clinical Neurophysiology, 88, 123-130.
Kraus N., McGee T.J., and Koch D.B. (1998) Speech sound representation, perception, and plasticity : a neurophysiologie perspective. Audiology Neuro-Otlogy, 3, 168-182.
Kraus N., Micco A.G., Koch D.B., McGee T., Carrell T., Sharma A., Wiet R.J., and Weingarten C.Z. (1993b) The mismatch negativity cortical evoked potential elicited by speech in cochlear-implant users. Hearing Research, 65,118-124.
Leipp E. La machine à écouter. Essai de psycho-acoustique. Ed Masson. 1977
Liberman MC. Auditory-nerve responses from cats raised in a low-noise chamber. J Acous Soc Am 1978 63:442455
Lina-Granade G. L'adaptation du système auditif périphérique chez l'humain. Thèse de Neurosciences, Lyon, 1996 Lorenzi C., Gallégo S., R.D. Patterson. Amplitude compression in cochlear implants artificially restricts the perception of temporal asymmetry. Brit J Audiol (1999)
Lorenzi C., Gallégo S., R.D. Patterson. Discrimination of temporal asymmetry in cochlear implantees. J Acoust Soc Am (1997), 102, 482-485
Lusted H, Shelton C, Simmons S. Comparison of electrode sites in electrical stimulation of the cochlea. Laryngoscope 1984 94:878-882
Mason S, Sheppard S, Garnham C, Lutman M, O'Donoghue, Gibbin K. Improving the relationship of intraoperative EABR threshold to T-level in young children receiving the Nucleus cochlear implant. ed. Advances in cochlear implant. Innsbruck: Verlag. 1994 44-49
Mc Dermott HJ, Mc Kay CM. Pitch ranking with nonsimultaneus dual-electrode electrical stimulation of the cochlea. J Acous Soc Am 1994, 96 155-162
Michelson RP. Electrical stimulation of human cochlear: A preliminary report. Arch Otolaryngol 1971 93(3):317 Miller CA, Abbas PJ, Brown CJ. Electrically evoked auditory brainstem response to stimulation of different sites in the cochlea. Hear Res 1993 66:130-42
Miller CA, Faulkner MJ, Pfingst BE. Functional responses from guinea pigs with cochlear implants. II Changes in electrophysiological and psychophysical measures over time. Hear Res 1995, 92 100-111
Miller CA, Woodruff KE, Pfingst BE. Functional responses from guinea pigs with cochlear implants. I Electrophysiological and psychophysical measures. Hear Res 1995, 92 85-99
Miskiewicz A, Scharf B, Hellman R, Meiselman C. Loudness adaptation at high frequencies. J Acous Soc Am 1993, 94 1281-1286
Moore BCJ, Glasberg BR. Gap detection with sinusoïds and noise in normal, impaired and electrically stimulated ears. J Acous Soc Am 1988 83:1093-1101
Moore JK. The human auditory brainstem as a generator of auditory evoked potentials. Hear Res 1987b 29:33-43 Moore JK. The human auditory brainstem: A comparative view. Hear Res 1987a 29:1-32
Morgon A, Aran JM, Collet L, Dauman R, Fraysse B, Freyss G, Pujol R, Sens A, Sterkers O, Pujol R. Le traitement de du son dans l'oreille interne. Pour la science, 1990, 154, 20-29
Moxon EC. Neural and mechanical responses to electric stimulation of cat's inner ear. Thesis. MIT. Cambridge, Mass, 1971
Nââtânen R. & Picton T. (1987) The N1 wave of the human electric and magnetic response to sound : a review and an analysis of the component structure. Psychophysiology 24, 375-425.
Nââtânen R., Gaillard A. and Mântysalo S. (1978) Early selective attention effect on evoked potential reinterpreted. Acta Psychologica 42, 313-329.
Nelson DA, Scmitz JL, Donaldson GS, Veimester NF, Javel E. Intensity discrimination as a fonction of stimulus level with electrical stimulation. J Acous Soc Am 1996 100:2393-2414
Oayoun MC. Traitement phonetique de la parole pour implants cochléaires. Thèse d'automatisme et traitement du signal, Paris, 1997
Patterson RD. The sound of a sinusoid : Spectral models. J Acous Soc Am 1994, 96 1409-1418
Patterson RD. The sound of a sinusoid : Time-interval models. J Acous Soc Am 1994, 96 1419-1428
Pfmgst B, Telman S, Sutton D. Operating ranges for cochlear implants. Mn Otol Rhinol Laryngol 1980 89(sup 66): 1-4
Pfingst B. Operating ranges and intensity psychophysics for cochlear implants. Arch Otolaryngol 1984 110:140-144 Pfmgst BE, Burnett PA, Sutton D. Intensity discrimination with cochlear implants. J Acous Soc Am 1993 94(3):1287-1294
Pfmgst BE, De Han DR,Holloway LA. Stimulus features affecting psychophysical detection thresholds for electrical
stimulation of the cochlea. I : Phase duration and stimulus duration. J Acous Soc Am 1991 90 1857-1866
Pfingst BE, Morris DJ, Miller AL. Effects of electrode configuration on threshold functions for electrical stimulation
of the cochlea. Hear Res 1995 85:76-84
Phillips AJ, Thornton ARD. Isochronic mapping of the auditory brainstem response : normative results. Brit J Audiol 1995, 29 335-346
Popelar J, Hartmann R, Syka J, Klinde R. Middle latency responses to electrical stimulation of the cochlea in cats. Hear Res 1995, 92 63-77
Ponton C.W., and Don M (1995) The mismatch negativity in cochlear implant users. Ear & Hearing, 16, 131-146. Ponton C.W., Don M., Waring M.D., Eggermont J.J., and Masuda A. (1993) Spatio-temporal source modeling of
evoked potentials to acoustic and cochlear implant stimulation. Electroencephalography and Clinical
Neurophysiology, 88, 478-493.
Raab DH, Taub HA. Click intensity discrimination with and without background masking noise. J Acous Soc Am 1969, 46, 965-968
Raab DH, Taub HA. Fluctuations in N1 amplitude in relation to click-intensity discrimination. J Acous Soc Am 1969, 46, 969-978
Rohen JW, Yokochi C. Anatomie Humaine. Atlas photographique de l'anatomie systématique et topographique. Vigot, 1991
Rose JE, Hind JE, Anderson DJ, Brugge JF. Phase-locked responses to low frequency tones in single auditory nerve fibers of squirrel monkeys. J Neurophysiol 1967 30:769-793
Rouanet H, le Roux B. Analyse des données multidimensionnelles. Ed Dunod. 1993
Sachs MB, Abbas PJ. Rate versus level functions for auditory-nerve fibers in cat: tone burst stimuli. J Acous Soc Am 1974 56:1835-1847
Sachs MB, Young ED. Encoding a stady-state vowels in the auditory-nerve :Representation in terms of discharge rate. J Acous Soc Am 1979 66, 470-479
Shallop JK, Beiter AL, Goin DW, Mischke RE. Electrically evoked auditory brain stem responses (EABR) and middle latency responses (EMLR) obtained from patient with the nucleus multichannel cochlear implant. Ear Hear 1990, 11 5-15
Shallop JK, VanDyke L, Goin DW, Mischke RE. Prediction of behavioral threshold and confort values for Nucleus 22-channel implant patients from electrical auditory brain stem response test results. Ann Otol Rhinol Laryngol 1991 100:896-898
Shallop JK. Objective electrophysiological measures from cochlear implants patients. Ear Hear 1993 14:58-63 Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science, 1995, 270, 303-304
Shannon RV. A model of threshold for pulsatile electrical stimulation of cochlear implants. Hear Res 1989 40:197204
Shannon RV. Detection of gap in sinusoïds and biphasic pulse trains by patients with cochlear implant. J Acous Soc Am 1989 85:2587-2592
Shannon RV. Multichannel electrical stimulation of the auditory nerve in man.I. Basic psychophysics. Hear Res 1983 11:157-189
Shannon RV. Temporal modulation transfer fonctions in patients with cochlear implants. J Acous Soc Am 1991 91:1256-1264
Simmons FB. Electrical stimulation of the auditory nerve in man. Arch Otolaryngol 1966 84:2-54
Smith DW, Finley CC, Honert C van den, Olszyk VB, Konrad KEM. Behavioral and electrophysiological responses to electrical stimulation in the cat.I. Absolute thresholds. Hear Res 1994 81:1-10
Smith L, Simmons FB. Estimating eighth nerve survival by electrical stimulation. Ann Otol Rhinol Laryngol 1983 92:19-23
Sohmer H, Feinmesser M. Cochlear action potentials recorded from the external ear in man. Ann Otol Rhinol Laryngol 1967 76:427-435
Spoendlin H, Schrott A. Analysis of the human auditory nerve. Hear Res 1989 43:25-38
Spoendlin H, Schrott A. The Spiral Ganglion and the Innervation of the Human Organ of Corti. Acta Otolaryngol (Stockh) 1988 105:403-410
Starr A, Brackman DE. Brainstem potentials evoked by electrical stimulation of the cochlea in human subjects. Ann Otol Rhinol Laryngol 1979 88:550-560
Stypulkowski PH, van den Honert C. Physiological properties of ellectrically stimulated auditory nerve. I Compound action potential recording. Hear Res 1984, 14 205-223
Tong YC, Clark GM. Absolute identification of electrical pulses rates and electrode positions by cochlear implant patients. J Acous Soc Am 1985 77(5):1881-1888
Townshend B, Coter N, van Compernolle D, White RL. Pitch perception by cochlear implant subjects. J Acous Soc Am 1987, 82 106-115
Tremblay K., Kraus N., Carrell T.D., and McGee T. (1997) Central auditory system plasticity : generalization to
novel stimuli following training. The Journal of the Acoustical Society of America, 102, 3762-3773.
Truy E., Gallégo S., Berger-Vachon C., A. Morgon. Multichannel cochlear implant in patients with ossified cochlea. Interest of the optimization of frequential selectivity of speech signal with Digisonic device. Proc. of IIIrd int. Symp. Transplants and Implants in Otology. Bordeaux (France), June 10-14 1995. (M. Portmann, P. Boudard, D. Portmann) 399-402
Truy E., Gallégo S., J.M. Chanal, Collet L., A. Morgon. Correlation between electrical ABR and perceptual thresholds in Digisonic cochlear implants users. Laryngoscope (1998), 108, 554-559
Truy E., Gallégo S., J.M. Chanal, Collet L., A. Morgon. Intérêt des potentiels évoqués auditifs électriques du tronc cérébral chez les patients porteurs de l'implant cochléaire Digisonic. Ann Otolaryngol Chir Cervicofac (1997), 114, 116-124
Truy E., JM Chanal, S Gallégo. Réglage des électrodes de l'enfant et de l'adulte. Rapport sur la prothèse auditive: Suppléance instrumentale de la surdité : les aides auditives (ed. SFORL), (1998), pp 361-372
Turner CW, Zwislocki JJ, Filion PR. Intensity discrimination determined with two paradigms in normal and hearingimpaired subjects. J Acous Soc Am 1989 86:109-115
Tyler RS, Parkinson AJ, Woodworth GG, Lowder MW, Gantz BJ. Performance over time of adult patients using the Inaired or Nucleus cochlear implant. J Acous Soc Am, 1997, 102, 508-522
Viemeister NF. Temporal modulation transfer fonctions based upon modulation thresholds. J Acous Soc Am, 1979, 66, 1364-1380
Wable J., S Gallégo, AM Jonas, I Roussillon, E Truy, Y Ormezzano, B Frachet, L Collet. Existe-t-il une relation entre les potentiels évoqués de latence tardive et la reconnaissance de la parole chez les implantés cochléaires? IVe C F Audiologie 7-8 décembre 1998, Clermont-Ferrand. Sous-presse
Wable J., S Gallégo, B Frachet. Adaptation chez les sujets porteurs d'un implant Digisonic. IVe C F Audiologie 7-8 décembre 1998, Clermont-Ferrand. Sous-presse
Wable J., T van Abbele, S Gallégo, Y Ormezzano, E Harboun-Cohen, M Papermann, I Roussillon, B Frachet. Evaluation des capacités de discrimination des implantés cochléaires à l'aide des potentiels évoqués de latence tartive. Sous-presse
Wable J., T. van Abbeele, Gallégo S., B. Frachet. Mismatch negativity: a tool for the assessment of stimuli discrimination in cochlear implant subjects. Accepté dans EEG Journal.
Waring MD. Auditory brain-stem responses by electrical stimulation of the cochlear nucleus in human subjects. Electroenceph clin Neurophysiol 1995 96:338-47
Waring MD. Electrically evoked auditory brainstem response monotoring of auditory brainstem implant integrity during facial nerve tumor surgery. Laryngoscope 1992 102:1293-5
Waring MD. Properties of auditory brainstem responses evoked by intra-operative stimulation of the cochlear nucleus in human subjects. Electroenceph clin Neurophysiol 1996 100 538-548
Zeng FG, Shannon RV.Loudness coding mechanisms inferred from electrical stimulation of the auditory system. Science 1994, 264 564-566
Zeng FG, Shannon RV.Loudness Growht in electrical stimulation. ed. Advances in cochlear implant. Innsbruck: Verlag. 1994 339-341

