II.2.2. Determination of the antioxidant potential of the
plant extracts
II.2.2.1. Determination of polyphenol content using Folin
ciocalteu method
Principle: This method is based on the
reduction of a phosphomolibdic-tungstinic chromogene by an antioxidant and a
change of colour with the absorbance measured at 750nm using a
spectrophotometer. This reagent constitute of a mixture of tungstinic and
phosphomolybdic acids. In alkali medium (sodium carbonate), it developed a blue
colouration of which the absorbance is measured at 750nm. Ethanol (0.3
ml) in the place of extract is used as the blank. The antioxidant activity is
expressed as the number of equivalents of catechin (Singleton and
Rossi, 1965).
- Preparation of the reagent
Folin-ciocalteu:
A stock solution Folin reagent of (10 ml) of concentration 2 N
was introduced in a conical flask of 100 ml and the volume adjusted with water
to 100ml so as to obtain a solution of 0.2 N.
- Preparation of catechine
standard:
Catechin (2 mg) was dissolved in 10 ml of methanol to obtain a
solution of concentration 1mM from which other solutions with diverse
concentration were prepared.
Procedure: The polyphenolic
concentration of the extracts was determined using folin-ciocalteu reagent
(sigma chemical Co St Louis, MO) diluted 10 times before use. To determine the
total polyphenol concentration, 10 ul of the hydrolyse extract was added in 1ml
of Folin solution diluted 10 times , after 30 minutes of incubation the
absorbance was measured at 750 nm using the spectrophotometer. Catechine was
used as a standard.
II.2.2.2. DPPH (1, 1-diphenyl-2-picrihydrazyl) antiradical
activity
Principle: DPPH is relatively stable nitrogen
centered free radical that easily accepts an electron or hydrogen radical to
become a stable diamagnetic molecule. DPPH antioxidant assay is based on the
ability of 1, 1-diphenyl-2-picryl-hydrazyl (DPPH), a stable free radical, to
decolorize in the presence of antioxidants. The DPPH radical contains an odd
electron, which is responsible for the absorbance at 517 nm and also for a
visible deep purple colour. When DPPH accepts an electron donated by an
antioxidant, the DPPH is decolorized, and can be quantitatively measured due to
the changes in absorbance (Katalinie et al.,
2003).
Procedure: 20 ul of none hydrolysed aqueous
extract was introduced in 2 ml of methanolic solution of DPPH (0.3 mM). After
30 minutes of incubation in the dark, the absorbance was measured with a
spectrophotometer at 517nm. A control was also made (DPPH with water only). The
percentage of inhibition of the DPPH radical by the specimen was calculated
using the formular of Yen and Duh, (1994) as follows:
% of inhibition = 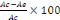 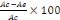
Where Ac is absorbance at time = 0 min and Ae is the
absorbance after 30 minutes of incubation.
II.2.3.The anti-á-amylase effect of Ficus ovata
extracts
Principle: The enzymatic activity was
measured by the colorimetric technique base on the disappearance of the
substrate in the reaction medium. This involved the inhibitory effect of
extract on pancreatic alpha-amylase ability to hydrolyse starch (starches
exists in the form of granules, composed of millions of molecules of
amylopectin and a higher number of molecules of amylose) into products
principally maltose that is colourless as seen in the reaction below. The
remaining substrate was thus quantified by adding iodine solution (iodine and
potassium iodide) in the reaction medium and the presence of a blue colour is
an indicator of the quantity of substrate remaining (Komaki et
al., 2003).
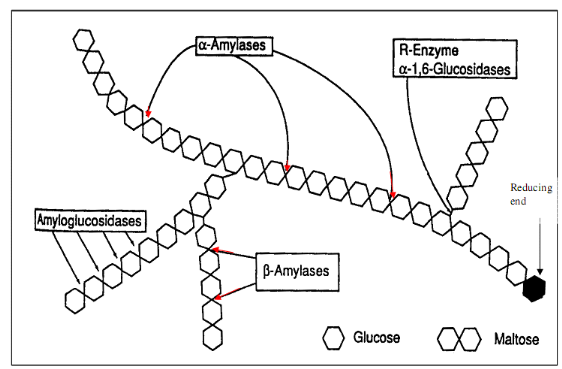
Figure 10 :
Mechanism of pancreatic alpha-amylase activity (Tormo et al,
2004)
Preparation of solutions
· Starch solution 10g/l (1%)
Irish (sigma) starch (5g) was introduced in a beaker
containing 100 ml of distilled water after which it was heated for 30 minutes
on a hot plate. The volume was completed to 500ml with distilled water
· Tris -HCl buffer pH 6.9 ;
0.05M
CaCl20.(56 g) of and 3.04 g of Tris was introduced
in a beaker containing 490 ml of distilled water, after complete
solubilisation, the pH was adjusted to 6.9 with dilute HCl.
· Iodine and Potassium Iodide
solution
One gram (1g) of potassium iodide and 100 mg of iodine are put
in a beaker containing about 490ml of distilled water. After complete
dissolution the solution was acidified to a pH of 1 with non title dilute HCl
and the volume was adjusted to 500 ml with distilled water.
· Preparation of a pig pancreatic
alpha-amylase
This was prepared to a concentration of 30ug/ml from the pure
pancreatic alpha-amylase of pig type V1-A (sigma).
· Evaluation of the activity of alpha-amylase
For each daughter solution there was an essay and a blank (no
substrate). A standard was also prepared where enzyme and substrate were
absent. 20 ul of á-amylase solution (30ug/ml) and the corresponding
volumes of Tris-HCl (0.05M, pH 6.9) and extract was introduced in each tube.
The mixture was pre-incubated at 37°C for 15 minutes and a fixed volume of
starch (1%) was then added in essay tubes followed by incubation at 37°C
for 15 minutes. The reaction was stopped by adding 2 ml of acidified iodine
solution and potassium iodide (pH 1). The intensity of colour of each tube was
determined against the blank through a spectrophotometer at an absorbance of
580nm. Methodology can be presented in a table as shown below.
Table VI: Methodology of á-amylase
inhibition
|
Tubes
|
standard
|
E0
|
BE0
|
E1
|
BE1
|
E2
|
BE2
|
E3
|
BE3...
|
E9
|
BE9
|
|
Enzyme (ul)
|
0
|
20
|
20
|
20
|
20
|
20
|
20
|
20
|
20
|
20
|
20
|
|
Extract (ul)
|
0
|
0
|
0
|
20
|
20
|
30
|
30
|
30
|
30
|
30
|
30
|
|
Buffer (ul)
|
1400
|
1380
|
1480
|
1350
|
1450
|
1350
|
1450
|
1350
|
1450
|
1350
|
1450
|
|
PREINCUBATION AT 37°C FOR 15 MINUTES
|
|
Starch (ul)
|
100
|
100
|
0
|
100
|
0
|
100
|
0
|
100
|
0
|
100
|
0
|
|
INCUBATION AT 37°C FOR 15 MINUTES
|
|
Iodine+KI(ml)
|
2
|
2
|
2
|
2
|
2
|
2
|
2
|
2
|
2
|
2
|
2
|
|
Final vol (ml)
|
3.5
|
3.5
|
3.5
|
3.5
|
3.5
|
3.5
|
3.5
|
3.5
|
3.5
|
3.5
|
3.5
|
The optical density OD for the final solutions was measured
using a spectrophotometer. The intensity of the colour of each tube is
determined against the blank.
Calculations of á- amylase
inhibition
- To get the quantity of the substrate
transformed
OD of the initial substrate (standard) - OD of the remaining
substrate (essay and blank)
This enables the elimination of non specific reaction between
the enzyme and the extract.
- Concentration of the transformed substrate
is gotten as follows
Concentration = 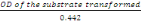 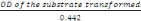
- Enzyme activity
This concentration was gotten after15 minutes
Therefore, Activity = 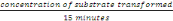 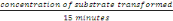
- Percentage inhibition
%inhibition = 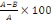 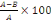
Where A is the decrease of absorbance in the absence of
extracts and B is the decrease of absorbance in the presence of extracts.
| 


