PREPARATION OF POLYSTYRENE WITH HIGHER Tg BASED ON
TRIPLE HYDROGEN BOND INTERACTION
MBULU AGALIA
MATERIALS SCIENCE
\u26085ÈÕ \u26399ÆÚ:
\u20108þ \u9675ð \u9675ð \u20061¾Å
\u24180Äê \u19968Ò» \u26376ÔÂ
\u20108þÊ® \u26085ÈÕ
Contents
Abstract...............................................................
......... .........2
Abstract in Chinese ...
................................................................4
Chapter 1 Introduction................ ...................
......5
1.1 Glass transition
temperature.............................................................5
1.2
Styrene and Maleimide
copolymer....................................................14
1.3 Melamine and chemistry
.............................................................23
1.4 Diaminopyridine
molecule..............................................................26
1.5 the thesis
work...........................................................................27
Chapter 2 Experimentation
Section.............................................31
2.1
Materials.................................................................................31
2.2
Instruments..............................................................................31
2.3 Synthesis of random copolymers of styrene and
maleimide......................32
2.4 Synthesis of Blends of Styrene/Maleimide copolymer and
melamine...........32
2.5 Synthesis of Blends of the copolymer and
2,6-diaminopyridine.................33
Chapter 3 Results and
discussion................................................34
3.1 Characterization of copolymer of styrene and
maleimide..........................34
3.2 Copolymerization of styrene and
maleimide........................................33
3.3 Blends of the styrene/maleimide copolymers and
melamine............................38
3.4 Blends of the copolymer and
2,6-diaminopyridine..................................43
Chapter 4
Conclusion.................................................................46
Reference...................................................................................47
PREPARATION OF POLYSTYRENE WITH HIGHER Tg BASED ON
HYDROGEN BOND INTERACTION
ABSTRACT
Polystyrene is one of a common polymer. But its glass
transition temperature (Tg) is only 100oC which leads to limit its
applications. In the thesis, improvement of its Tg was done via increase of its
chain interactions to restrict the chain flexibility.
Styrene was copolymerized with maleimide, imide of which act
as a hydrogen-bond interaction site in the copolymer, by free radical
polymerization method in DMSO solution at different temperature, and
different ratio of styrene to maleimide, and different reaction time, using
different amount of AIBN as an initiator. To make the imide distribute
randomly in the chains, maleimide was dropped slowly during the
polymerization. Melamine and diaminopyridine were selected to be as interaction
molecule because they can form the triple hydrogen bond with imide in the
copolymer. Addition of melamine or diaminopyridine into the copolymer results
in a dramatic increase of Tg. Polystyrene with imide molar concentration of
5.05% has Tg of 122oC in the presence of melamine, 22 oC
higher than polystyrene, which will extend application of polystyrene. At high
ratio of melamine to imide, two Tgs are observed, one is higher than
122oC but another much lower. The existence of two Tg is due to the
fact that free melamine is acting as plasticizer.
Diaminopyridine also increase Tg of polystyrene, but not
effectively than melamine owing to lack of crosslinking. In this case, the
blend of Diaminopyridine with polystyrene containing imide looks like a
copolymer of styrene with a big monomer, complex of maleimide and
Diaminopyridine.
The secondary interaction characteristics between melamine or
Diaminopyridine with polystyrene containing imide is confirmed by their blends
readily soluble in DMSO and in CH2Cl2.
Chapter 1 Introduction
1.1 Glass transition temperature
Many plastics lose their strength at relatively low
temperature. Continuous-service-temperature comparison of plastics reveals that
most common plastics can endure temperature more than 150oC when
under low or no stress. Glass transition temperature or Glass point
(Tg) is the point at which polymers act as glass or become viscous
liquids. That's a very important factor for polymers to evaluate their
processing and application performances.
The Glass transition is a reversible change that occurs when a
resin polymer is heated to a certain temperature (Tg), resulting in
a sudden change or transition from rigid polymer to a flexible, rubbery
material or a viscous liquid. When the polymer is cooled below this
temperature, it becomes hard and brittle, like glass. Certainly there are a few
polymers used above their Glass transition temperatures, however majority of
polymers are used below. Popular hard plastics like polystyrene
(Tg=100oC) is used below their glass transition
temperatures; that is its glassy state. Their Glass transition temperatures are
well above room temperature, both at around 100oC.
Rubbers elastomers like polyisoprene and polyisobutylene are
used above their Glass transition temperatures; that is in the rubbery sate
where they are soft and flexible.
The glass transition differs from the melting transition by
the fact that the former is a transition which happens to amorphous polymers
and the latter is a transition which occurs in crystalline polymers. But even
crystalline polymers will have some amorphous portions; this portion usually
makes up 40-70% of the polymer sample. This is why the same sample of a polymer
can have both a Glass transition temperature and a melting temperature. But
only the amorphous portion undergoes the glass transition and only the
crystalline portion undergoes melting. This change in mobility with temperature
happens because heat is really a form of kinetic energy.
The exact temperature at which the polymer chain undergoes
this big change in mobility depends on the structure of the polymer. A polymer
chain that can move around fairly easily will have a very low Tg, while one
that doesn't move so well will have a high one. The more easily a polymer can
move the less heat it takes for the chains to commence wiggling and break out
of the rigid glassy state and into the soft rubbery state. A given polymer
sample does not have a unique value of Tg because the glass phase
is not at equilibrium.
1.1.1 Factors governing Tg
The height of the glass-rubber transition temperature is, in
the first instance, governed by the competition between thermal motion and the
attraction forces between the chains.
The thermal motion is depend on the freedom of the chain to
undergo changes in conformation .When this freedom is higher, the chain is
subjected to a stronger thermal motion than a chain which, e.g. as result of
hindrance in rotation, is more rigid, the chain stiffness plays an important
role.
The primary criteria are:
-chain flexibility
-chain interactions
1.1.2 Chain flexibility
Higher chain stiffness results from a smaller number of
possible chain conformations; this can be caused by:
-greater stiffness of the main chain
-bigger side groups
-cross links
Some examples of the chain stiffness differences in the main
chain are:
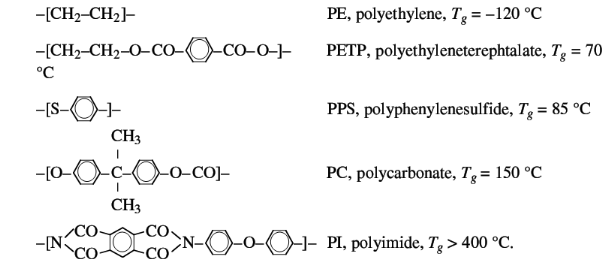
Some examples of the effect of side groups on the chain
flexibility are:
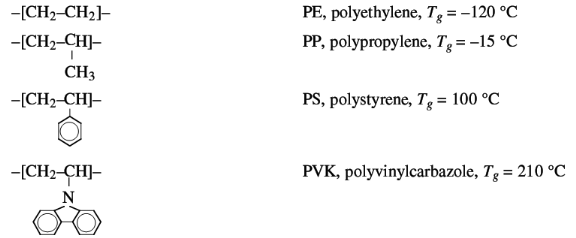
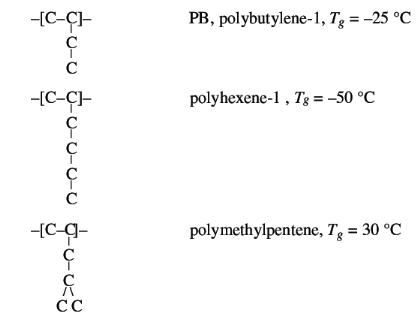
The increasing size of the side group effects a decrease of
chain flexibility and an increase of Tg.
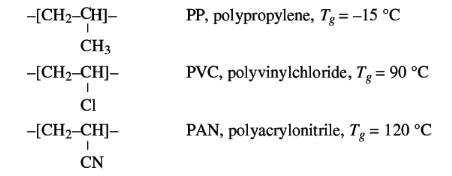
1.1.3 Chain interactions
The strongest of molecular interaction are the dipole forces.
Their effect on Tg is illustrated by the series PP, PVC and PAN, in which the
chain mobility hardly varies because the side groups are of about equal size,
but in which, in the order of sequence mentioned, the dipole interaction
increase.
Interaction can be decreased by increasing the distance
between the chains, for instance with long side chains, which lower Tg. This
effect appears to be greater than the increase of chain stiffness, as shown in
the examples below:
Approximate glass transition temperatures and melting point
of a few polymers are shown below:
Table1-1 glass transition temperatures of
common polymers
|
Polymer
|
LDPE
|
HDPE
|
PP
|
PVC
|
PS
|
PAN
|
PTFE
|
PMMA
|
PMMA
|
|
Tm(oC)
|
110
|
130
|
175
|
180
|
175
|
>200
|
330
|
180
|
30
|
|
Tg(oC)
|
-110
|
-110
|
-20
|
80
|
100
|
95
|
-110
|
105
|
-70
|
1.1.4 Intermolecular interaction
There are three types of intermolecular forces:
-Van Der Waals forces
-Dipole forces
-Hydrogen bond interactions
Although all such forces arise from the same fundamental
source i.e., interaction of negatively charged electrons and positively charged
nucleus yet they differ in magnitude, effective range and mode of operation.
Usually they are much smaller than the forces responsible for chemical
bonding.
1.1.5 Van Der Waals interactions
These interactions arise due to transfer polarization of
neutral molecules and are also known as London forces. Usually neutral
molecules have balanced number of negative electrons and positive charge on the
nucleus. Yet since electrons are in motion, the centre of density of negative
charge may not coincide with the centre of density of positive charge
continuously. A molecular thus acquires an electric dipole and can exert an
attraction for other similar molecules. Such interaction is known as van Der
Waals interaction.
A polarized molecule may induce the electric dipole in a
neutral molecule. However such polarized molecule continually reverts back to
neutral state and dipole is only transient. The greater the number of electrons
in a molecule and farther their distance from nucleus, the greater will be the
case of polarization and consently stronger Van Der Waals forces. These forces
vary inversely with the seventh power of the distance between molecules.
V.F. á 1/d7
Where V.F. is Van Der Waals forces and d is distance
separating the molecules. They are effective only over short intermolecular
distances.
1.1.6 Dipole-Dipole Interactions
Unequal sharing of electrons in covalent bonds results in
bonds dipoles and their magnitudes are indicated by the bond moments. As may be
expected the bond dipoles in different molecules attract each other resulting
in dipole-dipole interaction. These forces (D.F,) are governed by the
expression: D.F. á 1/d4
Where d is the distance between molecules, thus these forces
also are effectives only over short distances but have larger range than Van
Der Waals forces.
1.1.7 Hydrogen Bonds
It has been observed that when a hydrogen atom in a compound
is bonded to a highly electronegative atoms such as N, O, F, then marked
differences are observed in its usual properties like boiling point, solubility
etc. For example the boiling point of organic compounds usually increases with
increase in molecular weight but, though ethyl alcohol
C2H5OH (b.p.78.2o) and dimethyl ether
CH3-O-CH3 (b.p. -24.9 o) have the same
molecular weight, yet there is large difference in their boiling points.
The chemical properties of these compounds also differ as
compared to similar compounds not having hydrogen attached to N, O, F.
It is argued that when hydrogen is attached to such
electronegative atoms the bonding electrons are drawn strongly towards the
electronegative atom creating a dipole in the molecule. The hydrogen atom
therefore, acquires a small positive charge and becomes extraordinarily capable
of attracting a negatively charged atom of a molecule. This attraction results
in association of such molecules though the H-atom known as Hydrogen
Bond. This is represented by a dotted line. It has much less strength
than covalent bond and is essentially the result of electrostatic interactions,
delocalization effects and dispersion effects.
Hydrogen bonds are attractive interactions between a
positively charged hydrogen atom bonded to an electronegative element (the
donor: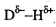 ), and a negatively charged atom with a lone pair of electrons (the
acceptor:
), and a negatively charged atom with a lone pair of electrons (the
acceptor: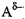 )
)
Table1-2 Functional group that can form
hydrogen bonds, arranged by element
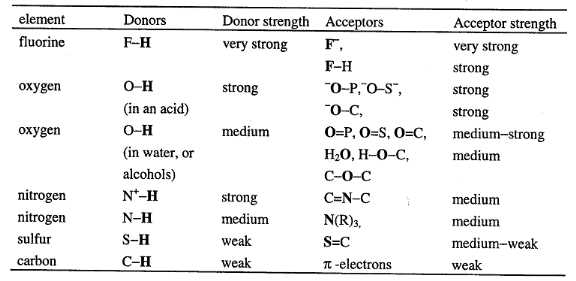
The strongest hydrogen bonding is formed between a strong
donor (like F-H and O-H in acid) and a strong acceptor.
The type of H-bonding resulting in association of two or more
molecules of the same or different compound is known as Intermolecular hydrogen
bonding. Intermolecular association trough hydrogen bond results in unusually
high boiling points of the liquids. Thus, the high boiling points of water,
alcohols, amines and acids as compared to monomeric molecules of comparable
molecular weight may be explained on the basis of H-bonding.
Intermolecular Hydrogen bonding is the formation of H-bonging
within the molecule itself. Ethylacetoacetate, salicylaldehyde and
o-nitrophenol are example of this type.
1.2 Styrene and Maleimide
copolymer
Polystyrene is one of a common polymer. It is
very easy to produce and proceed, so very cheap, and has majority of properties
for usage in common life. However, its glass transition temperature (Tg) is
only 100 degree Celsius, which leads to limit its applications.
Polymers with high glass transition temperature are attractive
for industrial polymer science because of their strong economic rewards that
may arise from their potential application [1]. As mentioned above,
two factors governs Tg of polymers, chain flexibility and chain interaction.
Copolymerization is a best way to change both of them. In the case of
copolymers, the final value of a given property; e.g. the melting point or the
glass transition temperature does depend on both of monomer structures and the
composition of them, also the others[2]. The existing methods used
to improve Tg of Polystyrene are the copolymerization and control of its
configuration. Incorporation of a few of another stiff monomer shows less
improvement in Tg of polystyrene, because the Tg is a function of content of
stiff monomer. More content of stiff monomer makes Tg of styrene copolymer
higher, meanwhile many good properties, for instance, stiffness, transparent,
and processing property, will be lost. Isotactic polystyrene has very high
Tg(above 220oC). However, it is difficult to process and,
furthermore, it is gotten by much more complicated coordination polymerization
route, not by the easy free radical polymerization.
Styrene molecule or derivates are chemically modified by free
radical polymerization to obtain new products with various potential
applications and properties [3]. Copolymerization of maleimides with
styrene provides the possibility of synthesizing higher and thermally stable
polymers. In addition to that the processability of maleimide polymer can also
be enhanced by the incorporation of more flexible units within the polymer
backbone [1].
Styrene-Maleimide copolymer (SMA) have been found to have
versatile applications in many industries ranging from aerospace to the
microelectronics field [2]. During the past several years many
reports and researches in the free radical copolymerization of styrene with
maleimide have emerged[2-5].
1.2.1 General description
When a polymer is made by linking only one type of small
molecule, or monomer, together, it is called a homopolymer. When two different
types of monomers are joined in the same polymer chain, the polymer is called a
copolymer. Two monomers A and B can be made into a copolymer in many different
ways.
When the two monomers are arranged in an alternating fashion, the
polymer is called an alternating copolymer:
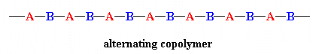
In a random copolymer, the two monomers may follow in any
order:
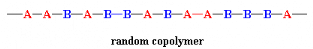
In a block copolymer, all of one type of monomer are grouped
together and all of the other are grouped together. A block copolymer can be
thought of as two homopolymers joined together at the ends:
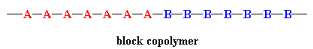
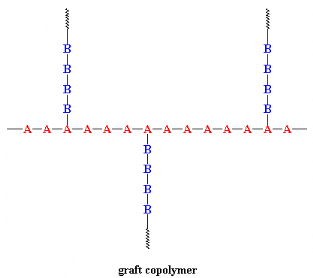
When chains of a polymer made of monomer B are grafted on to a
polymer chain of monomer A we have a graft copolymer:
Styrene, also known as vinyl benzene is an
organic compound
with the
chemical formula
C6H5CH=CH2. Under normal conditions, this
aromatic
hydrocarbon is an oily
liquid. It evaporates easily
and has a sweet smell, although high concentrations confer a less pleasant
odor. Styrene is the precursor to
polystyrene, an
important synthetic material. Styrene Monomer is the raw material for
polystyrene and EPS, accounting for approximately two thirds of total styrene
monomer production. The remaining styrene monomer is used as a feedstock in the
production of SAN, ABS and the unsaturated polyester resins, SBR and polymer
latex. Major downstream styrene markets (polystyrene and ABS) are under
pressure as a result of oversupply and interpolymer competition, although
rationalization of older, smaller styrenics units should help balance out the
market.
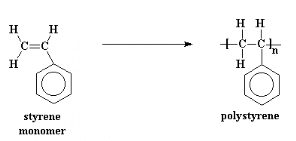
Maleimide is the
chemical compound
with the
formula
H2C2(CO)2NH. This unsaturated
imide is an important building
block in
organic synthesis.
The name is a contraction of
maleic acid and
imide, the -C(O)NHC(O)-
functional
group.
Fig1 Molecule of
Maleimide
Maleimide and its derivatives are prepared from
maleic anhydride by
treatment with
amines followed by
dehydration. A special feature of the reactivity of maleimides is their
susceptibility to additions across the double bond either by
Michael additions
or via
Diels-Alder
reactions.
A Copolymer is a polymer chain made up of two monomers units,
say A and B. When produced by copolymerization, the copolymer chain will
comprise a distribution of sequence lengths (S)which will depend upon the
monomer feed ratio [A]/[B] and the reactivity ratio (rA and
rB)which reflect the inherent tendencies of a radical to react with
its own monomer relative to the co-monomer.
Therefore, because of the feed ratio, the polymerization of
two or more monomers will result either to random copolymers, alternating
copolymer and block copolymers [6,7].
If a feed ratio of 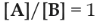 is taken, then the reactivity ratios will indicate the inherent tendency
of a system to produce particular sequence length distributions and hence the
characteristic average sequence lengths. As an example, for a perfectly
alternating copolymer, rA=rB=0 and the monomers alternate
along the chain. Alternatively, for a completely random copolymer
rA=rB=1 and SA=SB=2
is taken, then the reactivity ratios will indicate the inherent tendency
of a system to produce particular sequence length distributions and hence the
characteristic average sequence lengths. As an example, for a perfectly
alternating copolymer, rA=rB=0 and the monomers alternate
along the chain. Alternatively, for a completely random copolymer
rA=rB=1 and SA=SB=2
One of the most common and useful reaction for making polymers
is free radical polymerization.
It is used to make polymers from vinyl monomers, that is, from
small molecules containing carbon-carbon double bonds. Polymers made by free
radical polymerization include
polystyrene,
poly (methyl methacrylate),
poly (vinyl acetate) and branched
polyethylene.
Copolymerization of maleimide with styrene monomers results in
a polymerization mechanism occurring via a charge-transfer complex or via the
penultimate model, but it is obvious that polymerization of an electron rich
monomer (styrene) with an electron poor monomer (maleimide) leads to a,
predominantly, alternating copolymer [8,9].
Fig 1-1 charge transfer complex during
the polymerization of styrene-maleimide copolymer
Fig 1-2
Styrene-Maleimide Copolymers
1.2.2 The initiator of Styrene-Maleimide copolymer
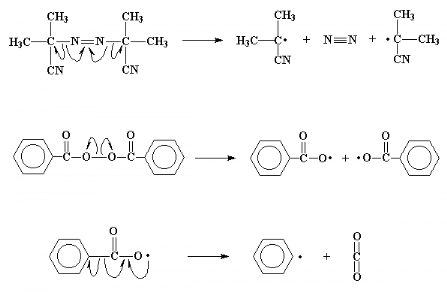
The whole process starts off with a molecule called an
initiator. This is a molecule like benzoyl peroxide or
2,2'-azo-bis-isobutyrylnitrile (AIBN).
What is special about these molecules is that they have an
uncanny ability to fall apart, in a rather unusual way. When they split, the
pair of electrons in the bond which is broken will separate. This is unusual as
electrons like to be in pairs whenever possible. When this split happens, we're
left with two fragments, called initiator fragments, of the original
molecule, each of which has one unpaired electron. Molecules like this, with
unpaired electrons are called free radicals.
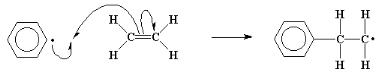
The carbon-carbon double bond in a vinyl monomer, like
ethylene, has a pair of electrons which is very easily attacked by the free
radical. The unpaired electron, when it comes near the pair of electrons, can't
help but swipe one of them to pair with itself. This new pair of electrons
forms a new chemical bond between the initiator fragment and one of the double
bond carbons of the monomer molecule. This electron, having nowhere else to go,
associates itself with the carbon atom which is not bonded to the initiator
fragment. You can see that this will lead us back where we started, as we now
have a new free radical when this unpaired electron comes to roost on that
carbon atom. This whole process, the breakdown of the initiator molecule to
form radicals, followed by the radical's reaction with a monomer molecule is
called the initiation step of the polymerization.
This process, the adding of more and more monomer molecules to
the growing chains, is called propagation.
Because we keep remaking the radical, we can keep adding more and
more ethylene molecules, and build a long chain of them. Self-perpetuating
reactions like this one are called chain reactions.
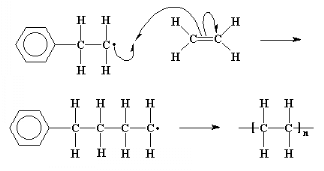
Coupling is one of two main types of termination
reaction. Termination is the third and final step of a chain-growth
polymerization.
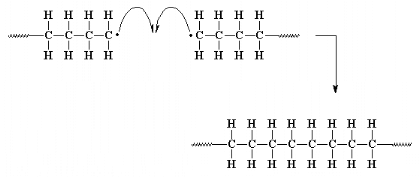
1.3 Melamine molecule and chemistry
As we known, the physical properties of polymers are
significantly altered upon addition of low molecular weight compounds, e.g. the
addition of plastizers, which increase chain mobility, and enhance the
processability of polymeric materials[4]. However, a low molecular
weight compounds is easy to distribute in polymers and have a strong
interaction with polymers in some cases. It's known that the used of secondary
interaction(such as hydrogen bond, ion-ion, dipole-dipole or Van Der Waals
forces ) is also a well-accepted strategy to enhance miscibility of immiscible
polymers, and miscible polymers mixture based on hydrogen
bonding[5]. Most significantly, secondary interaction for the
self-assembly of macromolecules has been investigated more recently in detail
for liquid crystallinity, rotaxanes, catenanes and amphiphilic structures
[5]. Recently, Lehn et al. introduced the concept of using
well-defined secondary interactions based on hydrogen bonding in the synthesis
of liquid crystalline polymer, in which the repeating units are linked by
triple-hydrogen bonding instead of by covalent bonding [6].
Ronald F.M. Lange et al. have studied the interaction between
the alternating copolymer of styrene/maleimide with unsubstituted melamine .
They observed that addition of melamine results in a dramatic decrease of
Tg up to melamine concentration of 20%(w/w) at which the
Tg remains constant at around 215 oC [5,7].
1.3.1 Structure of Melamine
Melamine is an organic base with the chemical formula
C3
H6
N6, with the
IUPAC name
1,3,5-triazine-2,4,6-triamine. It is only slightly soluble in water. Melamine
is a
trimer of
cyanamide. Like
cyanamide, it is 66%
nitrogen (by mass) and
provides
fire retardant
properties to resin formulas by releasing nitrogen when burned or charred.
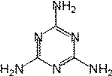
Fig 1-3 1,3,5-Triazine-2,4,6-triamine
1.3.2 Chemistry of Melamine
It is well-known that melamine forms a 1;1 crystalline complex
[5,7,12]with cyanuric acid. The structure of this complex is proposed to be an
infinite two dimensional lattice as is showing in the figure 5

Fig 1-4 infinite two dimensional lattices,
proposed for the 1:1 complex of melamine and cyanuric acid.
This 1:1 melamine-cyanuric acid lattice has been a source of
inspiration in the development of supramolecular chemistry and the theory of
secondary interaction [7,12].
Various model studies (e.g. complexation of unsubstituted
melamine with a low molecular weight imide as well as with various imides
containing polymers) have been performed to mimic this triple hydrogen bond
formation [12].
However, few attempts have been made to mimic this triple
hydrogen bond formation using high molecular polymeric materials. In order to
mimic this triple hydrogen bond formation only alternating copolymers of SMA
have been used in the synthesis of blend between SMA with either melamine or
2.4-diaminotriazine[12]
1.4 2,6-diaminopyridine molecule
2,6-diaminopyridine is analogous melamine. It can also form
the triple hydrogen bond with SMA.
Fig 1-5 Potential precursors of the triple
hydrogen bonded Styrene Maleimide couple
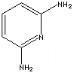
Fig 1-6 2,6-Diaminopyridine
PYRIDINE is a heterocyclic aromatic tertiary amine characterized
by a six-membered ring structure composed of five carbon atoms and nitrogen
which replace one carbon-hydrogen unit in the benzene ring
(C5H5N). The simplest member of the pyridine family is
pyridine itself. It is colorless, flammable, toxic liquid with a unpleasant
odor, miscible with water and with most organic solvents, boils at 115
oC. Its aqueous solution is slightly alkaline. Its conjugate acid is
called pyridinium cation, C5H5NH+, used as a
oxidation agent for organic synthesis. Pyridine is a base with chemical
properties similar to tertiary amines. Nitrogen in the ring system has an
equatorial lone pair of electrons that does not participate in the aromatic
pi-bond. Its aqueous solution is slightly alkaline. It is incompatible and
reactive with strong oxidizers and strong acids, and reacts violently with
chlorosulfonic acid, maleic anhydride, oleum, perchromates, b-propiolactone,
formamide, chromium trioxide, and sulfuric acid. Liquid pyridine easily
evaporates into the air. If it is released to the air, it may take several
months to years until it breaks down into other compounds. Usually, pyridine is
derived from coal tar or synthesized from other chemicals, mainly acetaldehyde
and ammonia.
Pyridine and its derivatives are very important in industrial
field as well as in bio chemistry. 2,6-Pyridinediamine is used as an
intermediate for the synthesis of analgesic drugs. Phenazopyridine is an
example derived from 2,6-Pyridinediamine.
1.5 The thesis work
The use of secondary interactions a well-accepted strategy to
enhance the miscibility of immiscible polymers, and miscible polymer mixtures
based on hydrogen bonding. This is result in interesting and enhanced polymeric
properties[12,13]. The use of hydrogen bonds offers the advantage
that they involve distinct donor and acceptor sites, and are very directional.
In that way they offer more possibilities for structural design than forces
that are symmetric and non-directional as e.g. ion-ion interactions.
In our case, we will improve Tg of polystyrene based on
secondary interactions. Knowledgably crosslinking can increase Tg of polymers.
However, nobody tried to use the method because few crosslinkage in polystyrene
will lead to worse rheological property.
The arrangement of hydrogen bond donors and acceptors of
maleimide, which are involved in hydrogen bonding in the complex with melamine,
suggest that it should be possible to complex one melamine molecule to three
imides units.
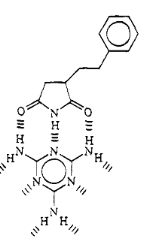
Fig 1-7 Proposed Structure of
Maleimide/styrene interactions with melamine
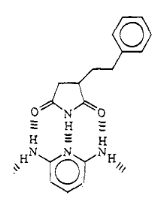
Fig 1-8 Proposed Structure of
Maleimide/styrene interactions with 2,6 diaminopyridine
In general, the binding strength of multiple hydrogen bonded
complexes is depending on the strength of the individual hydrogen bonds in the
array, and the number of hydrogen bond. Different hydrogen Donor(D) and
hydrogen acceptor (A) arrays can be obtained e.g. a triple bond( DDD-AAA array,
DDA-AAD array, DAD-ADA array)[12]. The DAD-ADA triple hydrogen bond array is
frequently used in organic chemistry due to its synthetic availability.
The use of a hydrogen bonding unit possessing two or more
interaction sites should result in network formation. It is a new, simple,
economical method to prepare polystyrene with higher Tg based on hydrogen
bonding crosslinkage between melamine and imide in the polystyrene prepared by
free radical copolymerization of styrene and few amount of maleimide. The
thermoreversible properties of hydrogen bond make the polystyrene have good
rheological properties with higher Tg.
In order to reveal the interaction between imide and melamine,
we used 2,6-diaminopyridine to replace melamine to complex with imide from SMA.
Both of them should complex with imide by DAD-ADA arrays shown in the Fig.1-7
and Fig.1-8.
Chapter 2 Experimental
2.1 Materials
Styrene was distilled under vacuum and stored in a freezer
until use. Maleimide 2,6-diaminopyridine were used commercially. AIBN
(2,2'-azobutironitrile) was recrystallised from methanol before use. DMSO was
purified and dried by standard techniques before use. CH3OH and
CH2Cl2 were used commercially.
2.2 Instruments
1H-NMR of copolymer samples were taken in
CDCl3 on a Bruker 600 MHz spectrophotometer with DMSO as a solvent.
The glass temperature transition was determined by differential scanning
calorimeter (DSC) and was performed on a Perkin Elmer Pyris 1 under nitrogen
with a scan rate of 10o C/min. The glass temperature transition Tg
is the midpoint in the heat-capacity change.
2.3 Synthesis of random copolymers of styrene and
maleimide
Copolymer of styrene and maleimide were prepared by a free
radical copolymerization using AIBN as the radical initiator and DMSO as
solvent. The synthesis of random copolymers of styrene and maleimide (Fig. 2-1)
which can serve as an example was performed as follow:
Styrene and Maleimide solution was prepared in DMSO. Styrene
solution was added with AIBN (80% w/w of the total monomer concentration) in a
3-necked round-bottomed flask equipped with a reflux condenser, mechanical
stirrer and nitrogen inlet (septum and a long needle). Maleimide solution was
added dropwise with AIBN (20% w/w of the total monomer concentration) shortly
and slowly. The polymer solution was precipitated in water and the resulting
polymer was dissolve in CH2Cl2 and precipitated in
CH3OH, then dried in vacuum at 60oC.
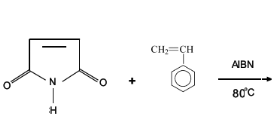
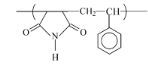
Fig 2-1 Synthesis Copolymerization of
Styrene and Maleimide
2.4 preparation of Blends of Styrene/Maleimide
copolymer and melamine or 2,6-diaminopyridine
Co-precipitation method was used to blend melamine or
2,6-diaminopyridine with imides containing copolymers. A
series of experiments was done to blend Styrene-Maleimide copolymers with
melamine or 2,6-diaminopyridine. Copolymers based on
styrene-maleimide were mixed homogenously with various amounts of melamine and
carefully dissolved in DMSO for 30 minutes, then co-precipitated in water. The
resulting polymer was dissolve in CH2Cl2 and precipitated
again in CH3OH then dried in vacuum at 50oC. After
filtration, washing and drying the yield of the blend was determined
[7].
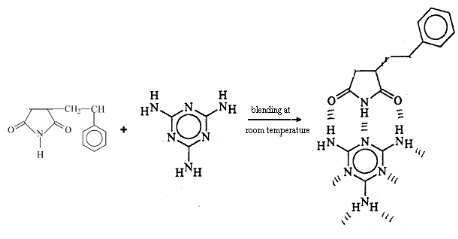
Fig 2-2 blend of Styrene-Maleimide and
Melamine
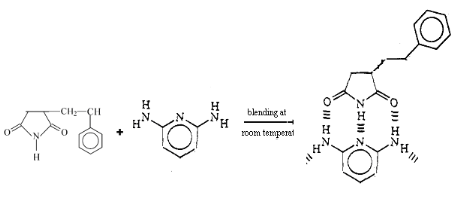
Fig 2-3 Blend of Styrene-Maleimide and
2,6-diaminopyridine
Chapter 3 Results and discussion
3.1 Characterization of copolymer of styrene and
maleimide
3.1.1 1H-NMR Spectra
In order to characterize the random styrene-maleimide
copolymer, 1H-NMR measurement was performed on a Bruker 600 MHz
spectrophotometer with DMSO as a solvent.
A typical 1H-NMR spectrum of copolymer Maleimide
Styrene is shown in figure 3-1.
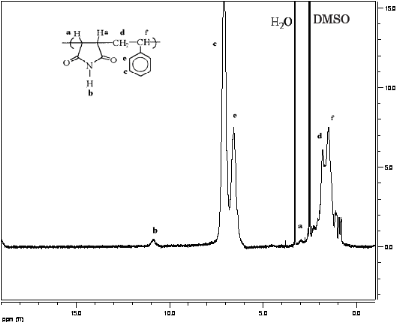
Fig 3-1 1H-NMR spectrum of random
copolymer Styrene-Maleimide at ratio 18.8/1
The characteristic spectrum was found for different ratio of
Styrene-Maleimide
The 1H-NMR spectrum shows the expected resonance
for the aromatic protons of polystyrene (c and e; ä~6.0-7.6 ppm proton
signals of the -CH= group of Styrene ) and imine protons of Maleimide
(b; ä~ 11.2ppm).
The broad signals from 1 to 3 ppm are
assignable to CH (a and f) and CH 2
(d) protons of the main chain. Therefore, this spectrum
confirms the presence of Maleimide-styrene copolymer [15].
All of the 1H-NMR spectra of random copolymer of
Styrene-Maleimide at different compositions have shown the same characteristics
bands.
3.1.2 Copolymer composition
1H NMR spectroscopic analysis has been established
as a powerful tool for the determination of copolymer compositions because of
its simplicity, rapidity and sensitivity. The average composition ratio of the
copolymer samples was determined from the corresponding 1H NMR
spectra. The assignment of the resonance peaks in the 1H NMR
spectrum leads to the accurate evaluation of the content of each kind of
monomeric unit incorporated into the copolymer chains.
Thus, the Copolymer ratio of SMA was calculated by measuring the
integrated peak areas of aromatic protons of styrene unit (signal c and e) and
imine proton of Maleimide (signal b). Let B be the integrated peak areas of
aromatic protons of styrene monomer and A is the integrated peak areas of imine
proton of Maleimide. The polystyrene unit contains five aromatic protons active
in resonance and the maleimide unit contains one imine proton, the following
expression is used to determine the composition of copolymer:

Table 3-1 Calculation of
integrated peak area of protons and mole compositions of copolymers
I-V
|
Copolymer
|
IAromatic
|
IImide
|
A
|
B
|
[Styr]/ [Mal]
|
|
I
|
28.832
14.843
|
1.000
|
1.000
|
43.675
|
8.7
|
|
II
|
1.000
0.5473
|
0.0233
|
0.0233
|
1.5473
|
13.2
|
|
III
|
1.000
0.5741
|
0.0167
|
0.0167
|
1.5741
|
18.8
|
|
IV
|
1.000
0.5581
|
0.0122
|
0.0152
|
1.5581
|
25.5
|
|
IV
|
1.000
0.3540
|
0.0153
|
0.0153
|
1.3540
|
17.7
|
I
Aromatic Integrated peak area of aromatic protons of
styrene unit.
Imide Integrated
peak area of an imine proton of Maleimide.
3.2 Copolymerization of styrene and maleimide
The random copolymer styrene-maleimide was easily obtained by
radical polymerization in DMSO using AIBN as the initiator. To control the
content of maleimide in copolymer, we performed the synthesis at different
conditions to copolymerize Maleimide and Styrene, and obtained copolymers with
identical main chain structure but with a different yield from 37.3 % to 89%.
As we mentioned before, Maleimide and Styrene could constitute the charge
transfer complex, so we chose dropwise addition of maleimide during the
polymerization.
Table 3-2 Effect of styrene/maleimide ratio on
yield
|
Experience
|
[Styr]/[Mal]
|
Reaction time/h
|
Temp/oC
|
Masse AIBN/%
|
Monomer Conc./M
|
Yield/%
|
[st]/[imide]
|
|
1
|
10/1
|
6
|
80
|
5
|
0.0476
|
39.4
|
8.7/1
|
|
2
|
15/1
|
44.5
|
13.2/1
|
|
3
|
20/1
|
80.0
|
18.8/1
|
|
13
|
30/1
|
89.0
|
25.5/1
|
Table 3-3 Effect of initiator on yield
|
Experience
|
[Styr]/[Mal]
|
Reaction time/h
|
Temp/ oC
|
Masse AIBN/%
|
Monomer Conc./M
|
Yield /%
|
|
4
|
20/1
|
6
|
80
|
1
|
0.0476
|
37.3
|
|
5
|
2
|
48.8
|
|
6
|
4
|
59.8
|
|
3
|
5
|
80.0
|
Table 3-4 Effect of temperature on yield
|
Experience
|
[Styr]/[Mal]
|
Reaction time/h
|
Temp/oC
|
Masse AIBN/%
|
Monomer Conc./M
|
Yield /%
|
|
7
|
20/1
|
6
|
60
|
5
|
0.0476
|
20.8
|
|
8
|
70
|
40.0
|
|
3
|
80
|
80.0
|
|
9
|
90
|
85.0
|
Table 3-5Effect of reaction time on yield
|
Experience
|
[Styr]/[Mal]
|
Reaction time/h
|
Temp/oC
|
Masse AIBN/%
|
Monomer Conc./M
|
Yield /%
|
|
10
|
20/1
|
4
|
80
|
5
|
0.0476
|
67.0
|
|
11
|
5
|
72.0
|
|
3
|
6
|
80.0
|
|
12
|
8
|
81.0
|
From these results we conclude that the ratio of the
copolymerization affects much more the yield.
Table 3-2 show that the yield of the copolymer increase with
the ratio of [Styrene]/[Maleimide]. That indicates maleimide introduction slows
the polymerization rate of styrene. However, the ratio of [St]/[imide] in the
copolymer is less than their monomer ratio counterpart, which means Maleimide
is easy to copolymerize with styrene.
Table 3-5 indicates that time is a parameter very important in
the copolymerization. As we described above, maleimide was added to the
solution shortly and slowly to avoid the formation of alternative copolymers
and to lead predominantly to the formation of random copolymer. To extend the
time reaction favors the formation of random copolymer. There is no
improvement in yield at reaction time of over 6hrs. that indicates effect of
maleimide on copolymerization is negligible. Table 3-3 and 3-4 have shown
respectively an increase of yield when the initiator and the temperature are
changed drastically. This result is demonstrated by the fact that the
copolymerization reaction is depending of the amount of initiator used and the
temperature required to activate the copolymerization.
3.3 Blends of the styrene/maleimide copolymers and
melamine
DSC curves of the blends of the copolymers
I-IV(as shown in Table 3-1) with melamine are
shown in Fig 3-2 and their Tg values are presented in Table3-6.
Table 3-6 Effect of imide /melamine ratio on
Tg
|
Experience
|
[imide]/[Mela]
|
[Styr]/ [Mal]
|
Tg/oC
|
|
F
|
1/1
|
18.8/1
|
103.3
|
|
G
|
1/2
|
113.8
|
|
H
|
1/3
|
122.0
|
|
I
|
1/4
|
125.6
105.5
|
|
J
|
1/5
|
128.0
78.0
|
|
K
|
1/10
|
130.0
90.0
|
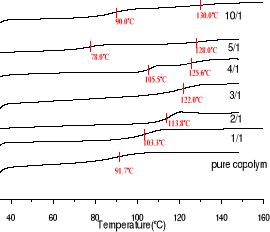
Fig 3-2 DSC traces of blends with different
molar concentration ratio melamine to imide in the copolymer with maleimide
molar concentration of 5.05 %
As shown in Fig3-2 and in Fig3-3, addition of
melamine results in a dramatic increase of Tg up to melamine concentration
ratio of 3 times to imide in the copolymer which correspond to Tg equal
122oC. In this case, Tg is 30oC higher than the pure
copolymer. At the melamine:imide ratio of 10:1, even 40oC is reached
with Tg of 130oC. However the more addition of melamine results in
presence of two value of Tg from the melamine: imide ratio of 4:1 to of 10:1.
It well known there is triple-hydrogen bonding between melamine and imide unit.
Recently, Ronald F. M [5,7,12] proposed that one melamine molecule interacts
with three imide units, leading to a three-dimensional hydrogen bonded
network.
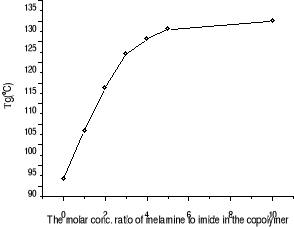
Figure 3-3 Dependence of Tg on the
ratio of melamine to imide in the copolymer
Tg increase of our copolymer in presence of melamine
attributes to this kind of crosslinkage restricting the motion of polystyrene
segments. The more crosslinkage in the blend corresponds to the higher Tg of
the blend. However, Maleimide contains in our copolymers is 20 times less than
Ronald's, and randomly distribute along the chain, which restricts the imides
together to interact with melamine. Therefore, more melamine is needed to build
a crosslinking site. As Figure 3-3 shown, melamine:imide ratio elevation from
3:1 to 10:1 still increases the Tg although slowly, suggesting crosslinkage
density still increases. From the melamine: imide ratio of 4:1 to of 10:1, the
presence of another Tg at lower temperature in case of melamine: imide ratio of
4/1, 5/1 and 10/1 is reasonably explained as the presence of free melamine
which acts as a plasticizer.
In order to increase crosslinkage sites, we prepared a series
of blends with different styrene/maleimide ratios. The results were given in
Table 3-8 and Fig 3-4.
Table 3-7 Effect of styrene/maleimide ratio on
Tg
|
Experience
|
[Styr]/ [Mal]
|
Tg/oC
|
|
A
|
polystyrene
|
100.1
|
|
B
|
8.1/1
|
84.6
|
|
C
|
13.2/1
|
87.4
|
|
D
|
18.8/1
|
91.7
|
|
E
|
25.5/1
|
93.0
|
Table 3-8 Effect of styrene/maleimide ratio on
Tg
|
Experience
|
[Styr]/ [imide]
|
[imide]/[Mela]
|
Tg/oC
|
|
L
|
8.1/1
|
1/5
|
106.9
|
|
M
|
13.2/1
|
123.3
|
|
N
|
18.8/1
|
128.0
78.0
|
|
O
|
25.5/1
|
146.2
89.0
|
|
P
|
8.1/1
|
1/3
|
109.0
|
|
Q
|
13.2/1
|
113.0
|
|
H
|
18.8/1
|
122.0
|
|
R
|
25.5/1
|
127.0
74.0
|
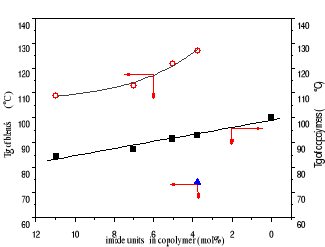
Figure 3-4 Dependence of Tg on imide contents
in copolymer (black spot)
Blends with molar ratio of melamine to imide 3:1(red spot)
Figure 3-4 reveals that Tg of copolymer decreased in a linear
function with maleimide content due to the flexibility of maleimide units.
However, Tg of all blends with melamine: imide ratio of 3:1 is much higher, at
least 25C, than a correspondent copolymer, and is an exponential decay
relationship with imide content. Blend of copolymer with the fewest imide
content([styrene]/[imide] 25.5/1) has the highest Tg, 127oC.
At the same melamine:imide ratio of 3:1, melamine isn't enough
to saturate imide units in the blends with higher imide content. However,
melamine is too much to complex with imide unit in the blend with imide content
of 25.5/1. It is confirmed by appearing another Tg at 74 oC. So, Tg
of blends with higher imide content must be much higher if more melamine is
used. With melamine: imide ratio of 5:1, we prepared a series of blends and
tested their Tg, shown in Table 3-8. their Tgs are much higher than ones of
blends with melamine: imide ratio of 3:1.
Moreover, in the procedure of preparation of blends, it is
observed that blends are readily soluble in DMSO, even in
CH2Cl2, this indicate that crosslinling are present in
our blends and is based on secondary interactions.
3.4 Blends of the copolymer and 2,6-diaminopyridine
In order to evaluate the effect of crosslinking of melamine on
the Tg of blend, we prepared a series of blend of Diaminopyridine(DAP) and
Styrene/Maleimide copolymer with different styrene/maleimide ratios. DAP is
analogue structure to melamine. They both can form a complex with imide through
a triple hydrogen bonds in the manner of DAD-ADA arrays, however without
crosslinkage is formed in the blends of DAP and Styrene/Maleimide copolymer
owing to difference of DAP and melamine in structure, as showed in Fig. 1-7 and
Fig.1-8.
DSC curves of the blends of Diaminopyridine and
Styrene/Maleimide copolymer are shown in Figure 3-5 and their
Tg values are presented in Table3-9.
Table 3-9 Effect of imide/Dap ratio on Tg
|
Experience
|
[imide]/[Dap]
|
[Styr]/ [imide]
|
Tg/oC
|
|
control
|
1/0
|
17.7/1
|
94.2
|
|
S
|
1/1
|
95.7
|
|
T
|
1/2
|
103.9
|
|
U
|
1/3
|
105.2
|
|
V
|
1/4
|
113.0
|
|
W
|
1/5
|
115.2
78.1
|
|
X
|
1/10
|
117.2
76.5
|
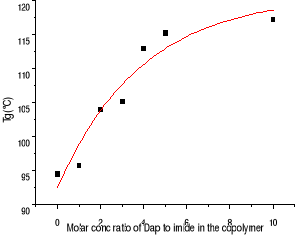
Figure 3-5 Dependence of Tg on the
ratio of DAP to imide in the copolymer
The dependence of Tg on the ratio of DAP to imide showed that
Tgs of blends are increasing with addition of DAP, as same as blends of the
copolymer with melamine. Single Tg is shown till DAP concentration ratio of 4
times to imide, which correspond to Tg equal 113oC . Then, two Tg is
found although one is still elevated. the behavior is found in blends of the
copolymer with melamine.
It is known there is triple-hydrogen bonding between DAP and
imide unit. Tg increase of our copolymer in presence of DAP attributes to this
kind of triple-hydrogen bonding existing in the blend and restricting the
motion of polystyrene segments, making the copolymer look like a copolymer of
styrene and complex of maleimide with DAP.
However, the Tg increase of the blends is lower ones of blends
of the copolymer with melamine. Contrary to the melamine, DAP molecule can not
have crosslinkng with imide unit. So the results confirm there are truly
crosslinking formation in blends of the copolymer with melamine.
Chapter 4 Conclusion
Tg of polystyrene, a common polymer, is dramatically elevated
based on crosslinkage between introduced imide units and melamine. Polystyrene
with imide molar concentration of 5.05% has Tg of 122oC in the
presence of melamine, 22 oC higher than polystyrene, which will
extend application of polystyrene. At high ratio of melamine to imide, two Tgs
are observed, one is higher than 122oC but another much lower. The
existence of two Tg is due to the fact that free melamine is acting as
plasticizer.
Diaminopyridine also increase Tg of polystyrene. In this case,
the blend looks like a copolymer of styrene with a big monomer, complex of
maleimide and Diaminopyridine. but increment is less than melamine owing to
lack of crosslinking between them.
The secondary interactions between melamine or Diaminopyridine
with polystyrene containing imide is confirmed by their blends readily soluble
in DMSO and in CH2Cl2.
References Notes
|
[1]
|
Kaj Backfolk et al;Determination of the glass transition
temperature of latex films:comparaison of various methods,''Department of
Physical Chemistry Akademi University ,Finland(2007)
|
|
[2]
|
Victor Morais,Random alternate and diblock
copolymers,Departement Materials,University Comlutense,Madrir,Spain(2005)
|
|
[3]
|
Xinliang Yu, Xueye Wang, Hanlu Wang, Aihong Liu and Cuili
Zhang , Journal of Molecular Structure: THEOCHEM, Volume 766, Issues 2-3,
15 August 2006, Pages 113-117
|
|
[4]
|
Xinliang Yuet al.;Prediction of the glass transition
temperatures of styrenic copolymers using a QSPR based on the DFT
method,College of Chemistry ,Xiangtan University ,people's of republic of
China(2006)
|
|
[5]
|
Kumar A, Galaev IY, Mattiasson B. Affinity precipitation of
á-amylase inhibiter from wheat metal by metal chelate affinity binding
using Cu (\u8545centsò) loaded copolymers of 1-vinylimidazole with
N-isopropy- acrylamide[J]. Biotechnol. Bioeng., 1998, 59: 693-704
|
|
[6]
|
Ronald lange and al.;Supramolecular Polymer Interaction based
on the alternating copolymer of styrene and maleimide,laboratory of organic
chemistry ,Eindhoven university,The Netherlands(1994); Supramolecular Polymer
Interaction using melamine, Laboratory of organic chemistry , Eindhoven
university,The Netherlands(1996)
|
|
[7]
|
Kotera,M;Lehn,J.chem.Soc.,Chem Comm.1994,197
|
|
[8]
|
Ronald lange ,Polymer blends based on the imidiaminotriazine
triple hydrogen bond ,Laboratory of organic chemistry , Eindhoven
university,The Netherlands(1997)
|
|
[9]
|
Mirzaagha babazadeh,thermal stability and high glass
transition temperature of 4-choloromethyl styrene polymers,Departement of
Applied Chemistry,islamic Azad university ,Iran(2006)
|
|
[10]
|
Bill meyer,;J.R.;Textbook of Polymer Science
3rdEd.;Wiley interscience,New york,USA(1984)
|
|
[11]
|
Melamine as a dietary nitrogen source for ruminants",
G.L.Newton and P.R.Utley, Journal of Animal Science, vol.47, p1338-44, 1978
|
|
[12]
|
E.E. Simanek, X. Li, I.S. Choi, G.M. Whitesides, "Cyanuric
Acid and Melamine: A Platform for the Construction of Soluble Aggregates and
Crystalline Materials", Comprehensive supramolecular chemistry, J.L. Atwood
ed., New York:Pergamon, Vol.9, 495 (1996).
|
|
[13]
|
Ronald lange and al.;Supramolecular polymer chemistry based on
melamine and maleimide-styrene copolymers,laboratory of organic chemistry
Eindhoven university,The Netherlands(1997)
|
|
[14]
|
Coleman ,Specific interaction and the miscibility of polymer
Blends ,Technomic,Lancaster(1991)
|
|
[15]
|
Koji Ishizu,Chisato Takashi?????Takeshi Shibuya and Satoshi
Unichida, Dept. of organic materilas and Macr.,Int. research Centre of
Macro.scienceTokyo Inst. of Tech.,Tokyo,Japan (2003)
|
|
|



