3.2 Copolymerization of styrene and maleimide
The random copolymer styrene-maleimide was easily obtained by
radical polymerization in DMSO using AIBN as the initiator. To control the
content of maleimide in copolymer, we performed the synthesis at different
conditions to copolymerize Maleimide and Styrene, and obtained copolymers with
identical main chain structure but with a different yield from 37.3 % to 89%.
As we mentioned before, Maleimide and Styrene could constitute the charge
transfer complex, so we chose dropwise addition of maleimide during the
polymerization.
Table 3-2 Effect of styrene/maleimide ratio on
yield
|
Experience
|
[Styr]/[Mal]
|
Reaction time/h
|
Temp/oC
|
Masse AIBN/%
|
Monomer Conc./M
|
Yield/%
|
[st]/[imide]
|
|
1
|
10/1
|
6
|
80
|
5
|
0.0476
|
39.4
|
8.7/1
|
|
2
|
15/1
|
44.5
|
13.2/1
|
|
3
|
20/1
|
80.0
|
18.8/1
|
|
13
|
30/1
|
89.0
|
25.5/1
|
Table 3-3 Effect of initiator on yield
|
Experience
|
[Styr]/[Mal]
|
Reaction time/h
|
Temp/ oC
|
Masse AIBN/%
|
Monomer Conc./M
|
Yield /%
|
|
4
|
20/1
|
6
|
80
|
1
|
0.0476
|
37.3
|
|
5
|
2
|
48.8
|
|
6
|
4
|
59.8
|
|
3
|
5
|
80.0
|
Table 3-4 Effect of temperature on yield
|
Experience
|
[Styr]/[Mal]
|
Reaction time/h
|
Temp/oC
|
Masse AIBN/%
|
Monomer Conc./M
|
Yield /%
|
|
7
|
20/1
|
6
|
60
|
5
|
0.0476
|
20.8
|
|
8
|
70
|
40.0
|
|
3
|
80
|
80.0
|
|
9
|
90
|
85.0
|
Table 3-5Effect of reaction time on yield
|
Experience
|
[Styr]/[Mal]
|
Reaction time/h
|
Temp/oC
|
Masse AIBN/%
|
Monomer Conc./M
|
Yield /%
|
|
10
|
20/1
|
4
|
80
|
5
|
0.0476
|
67.0
|
|
11
|
5
|
72.0
|
|
3
|
6
|
80.0
|
|
12
|
8
|
81.0
|
From these results we conclude that the ratio of the
copolymerization affects much more the yield.
Table 3-2 show that the yield of the copolymer increase with
the ratio of [Styrene]/[Maleimide]. That indicates maleimide introduction slows
the polymerization rate of styrene. However, the ratio of [St]/[imide] in the
copolymer is less than their monomer ratio counterpart, which means Maleimide
is easy to copolymerize with styrene.
Table 3-5 indicates that time is a parameter very important in
the copolymerization. As we described above, maleimide was added to the
solution shortly and slowly to avoid the formation of alternative copolymers
and to lead predominantly to the formation of random copolymer. To extend the
time reaction favors the formation of random copolymer. There is no
improvement in yield at reaction time of over 6hrs. that indicates effect of
maleimide on copolymerization is negligible. Table 3-3 and 3-4 have shown
respectively an increase of yield when the initiator and the temperature are
changed drastically. This result is demonstrated by the fact that the
copolymerization reaction is depending of the amount of initiator used and the
temperature required to activate the copolymerization.
3.3 Blends of the styrene/maleimide copolymers and
melamine
DSC curves of the blends of the copolymers
I-IV(as shown in Table 3-1) with melamine are
shown in Fig 3-2 and their Tg values are presented in Table3-6.
Table 3-6 Effect of imide /melamine ratio on
Tg
|
Experience
|
[imide]/[Mela]
|
[Styr]/ [Mal]
|
Tg/oC
|
|
F
|
1/1
|
18.8/1
|
103.3
|
|
G
|
1/2
|
113.8
|
|
H
|
1/3
|
122.0
|
|
I
|
1/4
|
125.6
105.5
|
|
J
|
1/5
|
128.0
78.0
|
|
K
|
1/10
|
130.0
90.0
|
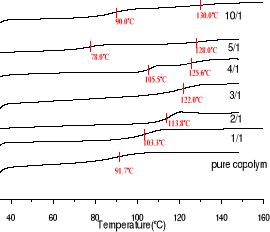
Fig 3-2 DSC traces of blends with different
molar concentration ratio melamine to imide in the copolymer with maleimide
molar concentration of 5.05 %
As shown in Fig3-2 and in Fig3-3, addition of
melamine results in a dramatic increase of Tg up to melamine concentration
ratio of 3 times to imide in the copolymer which correspond to Tg equal
122oC. In this case, Tg is 30oC higher than the pure
copolymer. At the melamine:imide ratio of 10:1, even 40oC is reached
with Tg of 130oC. However the more addition of melamine results in
presence of two value of Tg from the melamine: imide ratio of 4:1 to of 10:1.
It well known there is triple-hydrogen bonding between melamine and imide unit.
Recently, Ronald F. M [5,7,12] proposed that one melamine molecule interacts
with three imide units, leading to a three-dimensional hydrogen bonded
network.
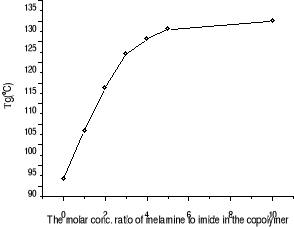
Figure 3-3 Dependence of Tg on the
ratio of melamine to imide in the copolymer
Tg increase of our copolymer in presence of melamine
attributes to this kind of crosslinkage restricting the motion of polystyrene
segments. The more crosslinkage in the blend corresponds to the higher Tg of
the blend. However, Maleimide contains in our copolymers is 20 times less than
Ronald's, and randomly distribute along the chain, which restricts the imides
together to interact with melamine. Therefore, more melamine is needed to build
a crosslinking site. As Figure 3-3 shown, melamine:imide ratio elevation from
3:1 to 10:1 still increases the Tg although slowly, suggesting crosslinkage
density still increases. From the melamine: imide ratio of 4:1 to of 10:1, the
presence of another Tg at lower temperature in case of melamine: imide ratio of
4/1, 5/1 and 10/1 is reasonably explained as the presence of free melamine
which acts as a plasticizer.
In order to increase crosslinkage sites, we prepared a series
of blends with different styrene/maleimide ratios. The results were given in
Table 3-8 and Fig 3-4.
Table 3-7 Effect of styrene/maleimide ratio on
Tg
|
Experience
|
[Styr]/ [Mal]
|
Tg/oC
|
|
A
|
polystyrene
|
100.1
|
|
B
|
8.1/1
|
84.6
|
|
C
|
13.2/1
|
87.4
|
|
D
|
18.8/1
|
91.7
|
|
E
|
25.5/1
|
93.0
|
Table 3-8 Effect of styrene/maleimide ratio on
Tg
|
Experience
|
[Styr]/ [imide]
|
[imide]/[Mela]
|
Tg/oC
|
|
L
|
8.1/1
|
1/5
|
106.9
|
|
M
|
13.2/1
|
123.3
|
|
N
|
18.8/1
|
128.0
78.0
|
|
O
|
25.5/1
|
146.2
89.0
|
|
P
|
8.1/1
|
1/3
|
109.0
|
|
Q
|
13.2/1
|
113.0
|
|
H
|
18.8/1
|
122.0
|
|
R
|
25.5/1
|
127.0
74.0
|
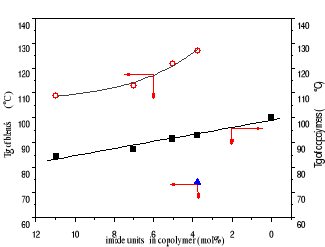
Figure 3-4 Dependence of Tg on imide contents
in copolymer (black spot)
Blends with molar ratio of melamine to imide 3:1(red spot)
Figure 3-4 reveals that Tg of copolymer decreased in a linear
function with maleimide content due to the flexibility of maleimide units.
However, Tg of all blends with melamine: imide ratio of 3:1 is much higher, at
least 25C, than a correspondent copolymer, and is an exponential decay
relationship with imide content. Blend of copolymer with the fewest imide
content([styrene]/[imide] 25.5/1) has the highest Tg, 127oC.
At the same melamine:imide ratio of 3:1, melamine isn't enough
to saturate imide units in the blends with higher imide content. However,
melamine is too much to complex with imide unit in the blend with imide content
of 25.5/1. It is confirmed by appearing another Tg at 74 oC. So, Tg
of blends with higher imide content must be much higher if more melamine is
used. With melamine: imide ratio of 5:1, we prepared a series of blends and
tested their Tg, shown in Table 3-8. their Tgs are much higher than ones of
blends with melamine: imide ratio of 3:1.
Moreover, in the procedure of preparation of blends, it is
observed that blends are readily soluble in DMSO, even in
CH2Cl2, this indicate that crosslinling are present in
our blends and is based on secondary interactions.
| 

