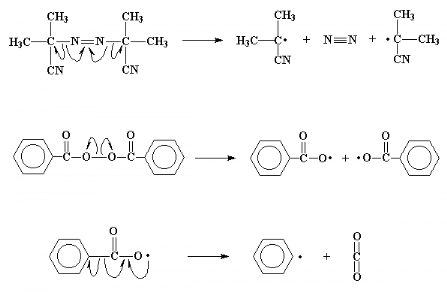
1.2.2 The initiator of Styrene-Maleimide copolymer
The whole process starts off with a molecule called an
initiator. This is a molecule like benzoyl peroxide or
2,2'-azo-bis-isobutyrylnitrile (AIBN).
What is special about these molecules is that they have an
uncanny ability to fall apart, in a rather unusual way. When they split, the
pair of electrons in the bond which is broken will separate. This is unusual as
electrons like to be in pairs whenever possible. When this split happens, we're
left with two fragments, called initiator fragments, of the original
molecule, each of which has one unpaired electron. Molecules like this, with
unpaired electrons are called free radicals.
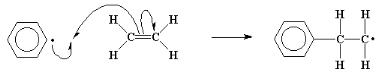
The carbon-carbon double bond in a vinyl monomer, like
ethylene, has a pair of electrons which is very easily attacked by the free
radical. The unpaired electron, when it comes near the pair of electrons, can't
help but swipe one of them to pair with itself. This new pair of electrons
forms a new chemical bond between the initiator fragment and one of the double
bond carbons of the monomer molecule. This electron, having nowhere else to go,
associates itself with the carbon atom which is not bonded to the initiator
fragment. You can see that this will lead us back where we started, as we now
have a new free radical when this unpaired electron comes to roost on that
carbon atom. This whole process, the breakdown of the initiator molecule to
form radicals, followed by the radical's reaction with a monomer molecule is
called the initiation step of the polymerization.
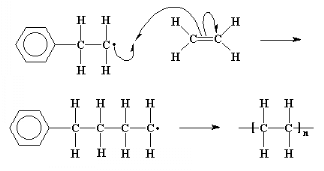
This process, the adding of more and more monomer molecules to
the growing chains, is called propagation.
Because we keep remaking the radical, we can keep adding more and
more ethylene molecules, and build a long chain of them. Self-perpetuating
reactions like this one are called chain reactions.
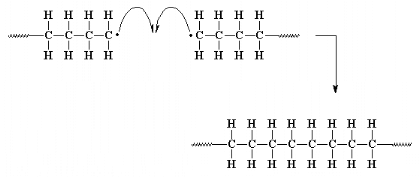
Coupling is one of two main types of termination
reaction. Termination is the third and final step of a chain-growth
polymerization.
| 

