4.2 pH effects and metal concentrations remained in
controls (blanks) 4.2.1 pH effects in blank samples
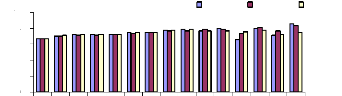
Figure 4.3 shows the variations of pH in blanks which are due
to elements contained in blank samples such as bacteria, phytoplanktons,
zooplanktons, also the variations of temperature can affects pH in blank
samples. The correlation between metal removal and the role of experimental
containers exists. The increasing or decreasing of pH in blank samples without
water hyacinth plants indicates that some elements of metal were fixed on the
internal surface of experimental buckets.
Oh
1 hr
3 hr
6 hr 10 hr 15 hr 21 hr 33 hr 57 hr 105 hr 177 hr 273 hr 393 hr
537 hr 705 hr
Exposure time (h)
pH
pH, 1mg/L pH, 3mg/L pH, 6mg/L
10
8
6
4
2
0
Figure 4.3: variations of pH in blank samples
4.2.2 Zinc concentrations remaining in blank
samples
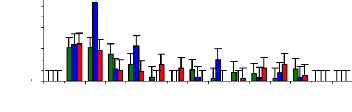
Figure 4.4 shows the trend of zinc concentration in blank water
samples at different
initial concentrations (1, 3 and 6 mg/L) and at
different periods of time. It was shown
that experimental small buckets may fix some trace elements of
zinc on internal surface of buckets or some trace elements were accumulated in
sediment because of the variation in metal concentration during the exposure
time. For 1 mg/L, the removal of zinc follows a linear trend of decreasing
concentration with the increasing of exposure time.
0.2
|
0.18
|
|
Zn2+, 1mg/L Zn2+, 3mg/L Zn2+, 6mg/L
|
|
0.16
|
|
Exposure time (h)
1 hr
Oh
6 hr
3 hr
33 hr
21 hr
15 hr
10 hr
57 hr
105 hr
177 hr
537 hr
393 hr
273 hr
705 hr
0.14
0.12
0.1
0.08
0.06
Conc. (mg/L)
0.04
0.02
0
Figure 4.4: Zinc conc. remaining in blank water samples over
time
4.2.3 Chromium concentrations remained in blank
samples
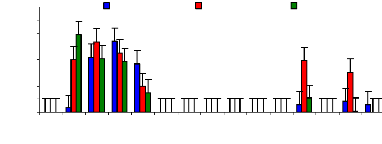
Figure 4.5 shows that chromium was quickly fixed on the
internal surface of experimental buckets and also due to phytoplankton,
zooplanktons in water samples. Some trace elements were analyzed in water from
1 to 10 hr only and after 177 hr, the internal surface releases chromium
concentration in water and other trace elements were accumulated in
sediment.
Exposure time (h)
Conc. (mg/L)
0.16
Cr6+,1mg/L Cr6+, 3mg/L Cr6+,6mg/L
0.14
0.12
0.1
0.08
0.06
0.04
0.02
0
Oh
1 hr
3 hr
6 hr
10 hr
15 hr
21 hr
57 hr
33 hr
105 hr
177 hr
273 hr
705 hr
537 hr
393 hr
Figure 4.5: Chromium conc. remaining in blank water samples
over time
4.2.4 Discussions of pH effects on metal concentrations
in blank samples
It was reported that pH variations affect metal concentrations
in blank samples. According to Barron et al. (1982), if metals are
present in wastewaters that contain hexavalant chromium, this chromium must be
reduced prior to metal removal. In general, hydroxides usually prove to be the
controlling species for adsorbing metal from industrial or domestic
wastewater.
The ANOVA analyses show that there is no effect of exposure
time to metal concentrations in blank samples for 1 and 3 mg/L (0.7 < 2.1;
1.6 < 2.1 respectively) but for 6 mg/L, the exposure time shows a
significant effect on metal concentrations (2.7 > 2.1). According to pH
variations, type of metal (zinc and chromium) and concentrations (1, 3 and 6
mg/L), there is a high significant difference (P = 0.05) observed during the
experiment.
4.3 pH variations and Zn(II) and Cr(VI) concentrations in
water
samples with water hyacinths
4.3.1 Variations of pH on metal removal by the
plants
The pH is an important parameter affecting the rate and the
extent of biosorption of metal ions onto bioadsorbents such as water hyacinth
plants. The variation of pH may affect the surface charge of roots of water
hyacinth plants and also the solubility of metal ions. Some metal ions are
known to be adsorbed or absorbed in the form of
hydroxides at high pH values such as pH>6. For this reason
the effects of initial pH on biosorption of Zn (II) and chromium (VI) ions onto
water hyacinth plants was investigated for the initial pH values equals to
6.7.
The variations of pH, zinc and chromium concentrations in
water samples with water hyacinth plants after 4 weeks of lab experiments in
which was observed an increasing in pH up to pH > 7.5 at 105 hr for 1 mg/L,
3 mg/L and 6 mg/L and then the situation changes after 105 hr of exposure to
metal.
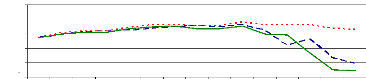
It was shown that pH variations affects metal removal during
the experiment. Figure 4.6 showed that the pH slightly increased from the
starting time (0 hr) (pH= 6.7) to 105 hr (pH= 7.64 to 7.86). However, after 105
hr of experiment, the pH decreased due to the saturation of adsorption sites,
so some H+ are released in water samples that caused the decreasing
of pH.
Zinc and chromium ions removal from solution was almost
completed within 105 hours for pH values above 6 and bellow 8 because of
adsorption. The pH of 6 is the critical point for zinc ions because of zinc
hydroxide adsorption or absorption. Therefore, it can be said that the optimum
pH for adsorption and absorption of zinc (II) and chromium (VI) ions by water
hyacinth plants in lab experimental set up is about pH =7.5.
|
Oh
|
1 hr
|
3 hr
|
6 hr
|
10 hr
|
15 hr
|
21 hr
|
33 hr
|
57 hr
|
105 hr
|
177 hr
|
273 hr
|
393 hr
|
537 hr
|
705 hr
|
Exposure time (h)
pH, 1mg/L pH, 3mg/L pH, 6mg/L
pH
4
9
8
7
6
5
Figure 4.6: pH variations in plant water samples over
time
4.3.2 Zinc concentrations remaining in water samples
after 4 weeks of experiment.
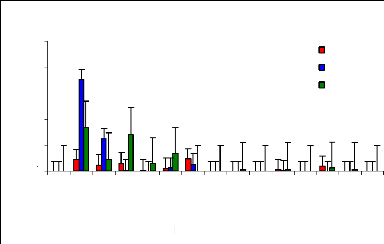
Figure 4.7 plotted out the remaining zinc concentration in
water samples after four weeks of lab experiments. It was observed that about
60% of zinc (II) was removed within 21 hours. Water hyacinths are effective
plants for zinc (II) ions removal form wastewater in the range of concentration
between 1 to 6 mg/L. Because the outer surfaces of water hyacinth roots are
negatively charged with some acetate groups and metal ions are positively
charged, roots attract metal ions, but when adsorption sites of roots are
saturated, it is expected that metal ions can be released in water samples. The
detention time must be determined. The passage in time influence the metal
removal, as the plant can once again release the metal ion back into the water
column when the adsorption sites become saturated. The decrease of pH in water
samples related to growing time is an important factor in metal absorption and
adsorption mechanisms by the plants. It is very clear that after 21 hr, little
trace elements of metal are still present in water samples with water hyacinth
plants.
0.25
Exposure time (h)
57 hr
Oh
1 hr
537 hr
6 hr 10 hr
177 hr
273 hr
705 hr
393 hr
105 hr
33 hr
21 hr
15 hr
3 hr
Zn2+, 1mg/L
Zn2+, 3mg/L
Zn2+, 6mg/L
0.2
0.15
Conc. (mg/L)
0.1
0.05
0
Figure 4.7: Zinc conc. remaining in water samples with water
hyacinth plants over time
| 


