|
JULY, 2019
THE UNIVERSITY OF BAMENDA
|
FACULTY OF HEALTH SCIENCES
|
|
DEPARTMENT OF CLINICAL SCIENCES
|
ETIOLOGIES, CLINICAL PRESENTATION AND
HOSPITAL
OUTCOME OF BACTERIAL MENINGITIS IN CHILDREN
AT THE PEDIATRIC
UNIT OF THE YAOUNDE -GYNECO -
OBSTETRIC AND PEDIATRIC HOSPITAL
A Dissertation submitted to the Department of Clinical
Sciences in the Faculty of Health
Sciences in partial fulfilment of the
requirements for the award of a Postgraduate
Diploma (DM) in Clinical
Sciences.
By
MAURANE EMMA NDJOCK MBEA
Registration
Number: UBa12H034
SUPERVISOR CO-SUPERVISOR
Prof. CHIABI ANDREAS Prof. ATANGA MARY
(c) Copyright by Maurane Emma Ndjock Mbea, 2019
All Rights Reserved
1
DECLARATION
I, Nlaurane Emma Ndjock Mbea, Registration N° UBaI2HO34,
Department of Clinical Sciences in the Faculty of Health Sciences, University
of Bamenda, hereby declare that, this work titled: "Etiologies, Clinical
Presentation And Hospital Outcome Of Bacterial Meningitis In Children At The
Pediatric Unit Of The Yaounde --Gyneco -- Obstetric And Pediatric Hospital" is
my original work. It has not been presented in any application for a degree or
any academic pursuit. 1 have sincerely acknowledged all borrowed ideas
nationally and internationally through citations.
|
Date 4 (t'A f q-cac)
|
|
|
|
|
Signature ~~ j
|
ii

iii
DEDICATION
TO MY PARENTS
CHANTAL
BAYEGUI Epse NDJOCK MBEA
AND
EMMANUEL NDJOCK MBEA
TO MY
SISTER
FINKE FICTIME YAN NDJOCK MBEA
iv
ACKNOWLEDGEMENT
I am most grateful to:
Thesis supervisor Prof CHIABI Andreas for his
outstanding teaching received during the course of the work. Professor, your
rigor and constant devotion for a work perfectly done forces our admiration.
Thesis co directors Prof NGUEFACK Seraphin
and Prof ATANGA Mary for their acceptance given to us
in agreeing to guide us in this work. Thank you once more for your availability
and dynamism.
Dean of the Faculty of Health Sciences of the
University of Bamenda for her constant advice and support given to us at any
given circumstances to all her students.
Pediatric Department of YGOPH for their
welcome and help during our study.
Formal Dean of the Faculty of Health Sciences
for his rigorous training he has given to us.
My parents Mr and Mrs Ndjock Mbea for their
love, moral and financial support they have been given me throughout the
realization of this work.
My family members: Mrs Finke Fictime Marie Louise
for her moral and financial support, my sisters Finke yan
and Bayegui Marie Louise for their prayers and moral
support.
Second batch of Faculty of Health Sciences of
the University of Bamenda and my friend Ngo Linwa Esther for
the exceptional 7 years they have made me gone through, together in struggle
and brighter days, thanks.
To all whose names are not cited, I say my most gratitude
towards any form of help from you, thanks.
GOD Almighty for the inspiration, mental and
physical strength and protection he gave me to be able to go through this work
successfully.
v
ABSTRACT
INTRODUCTION AND OBJECTIVES:
Meningitis is a term that describes the inflammation of
membranes (meninges) and/or cerebrospinal fluid that surrounds and protects the
brain and spinal cord. Bacterial meningitis remains a serious global health
problem, with World Health Organization estimating over 1.2 million of cases
worldwide each year. It still affects mostly children with significant
morbidity and mortality despite the presence of vaccins. Many studies have been
conducted out of Africa, in Africa and in Cameroon with an incidence of 1.54 in
2014, all emphasizing the continuous rising incidence, thus our main aim being
to: determine incidence, etiologies, clinical presentations, and describe the
hospital outcome of bacterial meningitis in children.
MATERIALS AND METHODS:
This was a retrospective cross sectional descriptive study
done at the pediatric unit of the Yaoundé Gyneco-Obstetric and Pediatric
hospital. Those included in the study were children admitted for meningitis
from the 1st of January 2014 till the 31st of December
2018 and aged from 1 month to 15 years. The sample size of 23 was calculated
from the Cochrane's formula, and there was consecutive search of files in the
archives for the following information: pathogens isolated and clinical
manifestation and hospital outcome of children with the disease. The data were
recorded using CS PRO 7.2(census and survey processing system) soft ware and
analysed using IBM SPSS 23.0(statistical package for the social sciences)
RESULTS:
The incidence of bacterial meningitis in children at the
Yaoundé Gyneco -Obstetric Hospital was 0.3% and the female sex was
predominant at 56% in the admissions. Streptococcus pneumoniae and
Neisseria meningitidis were the most common pathogens isolated; 63%
and 25% respectively. Children within the age group of < 12 months were the
most affected. Fever (95.3 %) and convulsion (60.5%) were the most common
presentations of meningitis at time of admission, while neck stiffness and
meningeal signs (Kerning and Brudzinski's signs) were present on clinical
examination at 20.9% and16.3% respectively. A mortality rate of 2.4% was
recorded against cure rate of 97.6%.
vi
CONCLUSION AND RECOMMENDATION:
The incidence of bacterial meningitis was 0.3% with
streptococcus pneumoniae being the most common pathogen identified,
and fever and convulsion as the most common presentations of meningitis. Neck
stiffness and meningeal signs were the most common signs on clinical
examination. There was a low mortality rate of 2.4 %.
Following our conclusions ,the ministry of public health is
recommended to look into vaccination campaigns in children all over the country
against infectious diseases especially meningitis. This could be done through
re-enforcement of information, education and communication of vaccination in
children.
vii
RESUME
INTRODUCTION ET OBJECTIFS:
La méningite est un terme qui décrit
l'inflammation des meninges et /ou le liquide céphalo-rachidien qui
entoure et protège le cerveau et la moelle épinière. La
méningite bactérienne reste un problème de santé
publique sérieuse, avec l'Organisation Mondiale de la Santé
l'estimant à plus de 1,2 million de cas dans le monde entier chaque
année. Elle affecte toujours les enfants, avec une morbidité et
une mortalité significative malgré la présence des
vaccins. Plusieurs études ont été faites hors d'Afrique,
en Afrique en général et au Cameroun avec une incidence à
1,54 en 2014, toutes mettant l'accent sur la monté des incidences
d'où le but de cette étude qui et de : déterminer
l'incidence, les étiologies, la présentation clinique, et le
devenir hospitalier de la méningite bactérienne chez les
enfants.
METHODOLOGIE:
Cette étude était
rétrospective-descriptive et a été faite à
l'Hôpital Gynéco-Obstétrique et Pédiatrique de
Yaoundé. Ceux inclus dans l'étude étaient les enfants
admis pour la méningite du 1er Janvier 2014 au 31
Décembre 2018 et âgés de 1 mois à 15 ans. La taille
d'échantillon était de 23 a partir de la formule de Cochrane et
y'avait une fouille consécutive de dossiers aux archives pour recueillir
les informations suivantes : germes isolées, présentation
clinique et devenir hospitalier chez l'enfant atteint de la maladie. Les
données ont été saisis a l'aide du logiciel CS PRO 7 .2 et
analysées avec le logiciel IBM SPSS 23.0
RESULTATS:
L'incidence de la méningite bactérienne
était de 0.3% à l'Hôpital Gynéco-Obstétrique
et Pédiatrique de Yaoundé avec une prédominance de genre
féminin à 56% à l'hospitalisation. Streprococcus
pneumoniae et Neisseria meningitidis étaient les germes
les plus isolés à 63 % et 25 % respectivement .Les enfants
âgés de < 12 mois étaient les plus affectés. La
fièvre et la convulsion étaient des présentations les plus
communes a la consultation, pendant que la raideur de la nuque (20.9%) et les
signes méningés(Signes de Kerning et de Brudzinski) (16.3%)
étaient les plus retrouvés comme signes à l'examen
clinique. Un taux de 2.4 % de mortalité était enregistré
contre un taux de 97.6% de guérison.
viii
CONCLUSION ET RECOMMANDATION:
L'incidence de la méningite était de 0.3 % avec
le Streptococcocus pneumoniae comme germe le plus isolé et la
fièvre et la convulsion étaient les présentations les plus
communes de la méningite. La raideur de la nuque et les signes
méninges comme signes les plus communs à l'examen clinique. Il
y'avait un taux bas de mortalité à 2.4%
Compte tenue de ces conclusions, le ministère de la
santé publique est recommandé de mettre un regard sur les
campagnes de vaccination des enfants dans tout l'étendu du territoire
contre les maladies infectieuses surtout sur la méningite. Le
renforcement de l'information, l'éducation et la communication des
vaccinations chez les enfants est nécessaire.
ix
TABLE OF CONTENTS
DECLARATION i
CERTIFICATION ii
DEDICATION iii
ACKNOWLEDGEMENT iv
ABSTRACT v
RESUME vii
TABLE OF CONTENTS ix
LIST OF TABLES xii
LIST OF FIGURES xiii
LIST OF ABBREVIATIONS AND SYMBOLS xiv
HIPPOCRATIC OATH xx
CHAPITRE ONE 1
INTRODUCTION 1
I.1) Background 1
I.2) Rationale And Justification 2
I.3 ) Main Objectives 2
I .3.1) Specific Objectives 2
I.5) Research Question 3
I.6 Conceptual Framework 3
CHAPTER TWO 4
LITERATURE REVIEW 4
Ii.1) General Overview 4
Ii.4) Pathophysiology Of Bacterial Meningitis 12
Ii.4.1) Bacterial Invasion 12
Ii.4.2) Inflammatory Response 14
Ii.4.3) Raised Intracranial Pressure 14
Ii.4.4) Neuronal Damage 15
Ii.5) Diagnosis Of Meningitis In Children 16
Ii.5.1) Clinical Diagnosis Of Meningitis In Children 16
Ii.5.2) Paraclinical Diagnosis Of Bacterial Meningitis 17
Ii.5.2.1) Lumbar Pucture And Csf Analysis 17
Ii.5.2.2) Complications Of Lumbar Puncture 19
Ii.5.3) Other Laboratory Investigations 19
Ii.6)
x
Current Treatment On Bacterial Meningitis In Children 21
Ii.6.1) Antibiotic Treatment 21
Ii.6.2) Adjuvant Therapy And Supportive Therapy 22
Ii.6.3) Fluid Restriction 22
Ii.7.2) Chemoprophylaxis 24
Ii.7) Complications Of Bacterial Meningitis In Children 24
Ii.8) Publications On Meningitis 26
CHAPTER THREE 29
MATERIALS AND METHODS 29
Iii.1) Study Design 29
Iii.2) Duration Of The Study 29
Iii.3) Study Setting 29
Iii.4) Sampling 29
Iii.5) Study Population 29
Iii.5.1) Inclusion Criteria 29
Iii.5.2) Exclusion Criteria 30
Iii.6) Sample Size 30
Iii.7) Materials 30
Iii.8) Methods 31
Iii.8.1) Administrative Authorizations And Ethical Clearance
31
Iii.8.2) Patient Recruitment 31
Iii.8.3) Data Management 33
Iii.8.3.1) Ethical Considerations 33
Iii.9.1) Human Resources 33
CHAPITRE FOUR 34
RESULTS 34
Iv.1) Incidence 34
Iv.2.3) Type Of Admissions 35
V.2.4) Distribution Of Patients According To Year Of Admission
36
Iv.3) Clinical Presentation Of Bacterial Meningitis 37
Iv.5) Etiologies Of Bacterial Meningitis 41
xi
CHAPITRE FIVE 47
DISCUSSION 47
V.1) Incidence 47
V.2) Clinical Presentation Of Patients 48
V.3) Hospital Outcome Of Bacterial Meningitis In Children
50
Conclusion 51
Recommendations 51
REFERENCES 53
APPENDICES
xii
LIST OF TABLES
Table I: Distribution of patients according
to gender 35
Table 2: Distribution of clinical
presentation according to symptoms. 37
Table 3: Distribution of neurological
clinical presentation according to signs 38
Table 4: Biochemical and cytological aspect
of csf analysis of patients at admission 39
Table 5: Biochemical and cytologic aspect of
csf analysis of patients at admission 40
Table 6: Distribution of pathogens according
to age 42
Table 7: Distribution of complications found
during admission 43
Table 8: Distribution of patients according
to outcome during admission 44
Table 9: Distribution of patients according
to sequelae at time of discharge 45
Table 10: Distribution of patients according
to treatment recieved for sequalae 46
xiii
LIST OF FIGURES
Figure 1: Meningitis Belt in West Africa [14]
4
Figure 2: Countries trained to conduct
surveillance for the Pediatric Bacterial Meningitis Surveillance Network, by
performance level* --- World Health
Organization African Region, 2008 [16] 5
Figure 3:
Causes of confirmed bacterial meningitis from eleven years of
active
surveillance in a Mexican hospital, 2005 -2016. [18] 6
Figure 4 : Pathogenesis of bacterial meningitis
[38] 15
Figure 5: Lumbar spine anatomy[41]. 17
Figure 6 : Distribution of patients according to
age groups 34
Figure 7: Distribution of patients according to
the type of admission. 35
Figure 8: Flow chart illustrating the incidence
per year at YGOPH. 36
xiv
LIST OF ABBREVIATIONS AND SYMBOLS
C D14 : Cluster of differentiation 14
CbpA : Choline binding protein A
CSF : Cerebro spinal fluid
E.coli : Escherichia coli
H.influenzae : Haemophilus influenzae
Hib : Haemophilus influenzae type b
Ibe A : Invasion of brain endothelium protein
A
Ibe B : Invasion of brain endothelium
protein
N.meningitidis : Neisseria meningitidis
OmpA : Outer membrane protein A
S .pneumoniae : Streptococcus pneumoniae
SDG : Sustainable development Goal
WHO : World's Health Organisation
YGOPH : Yaoundé-Gynaeco-Obstetric and
Pediatric Hospital
xvi
THE ADMINISTRATIVE STAFF OF THE UNIVERSITY OF
BAMENDA
Prof Sammy Beban Chumbaw Pro-Chancellor
Prof Theresia Akenji Vice-Chancellor
Prof Suh Cheo Emmanuel Deputy Vice-Chancellor in
charge of Teaching,
Professionalization and
Development of Information
and Communication
Technologies
Prof Agwara Moise Ondoh Deputy Vice-Chancellor in
charge of Internal Control and Evaluation
Prof Roselyn Jua Deputy Vice-Chancellor in
charge of Research,
Cooperation and relationship with the
Business world
Prof Banlilon Victor Tani Registrar
Prof Ghogomu Julius Numbonui Director of Academic Affairs
Dr Mbifi Richard Director of Administrative
Affairs
Prof Anong Damian Nota Director Students' Affairs
MrGiyohYerima Peter Director of Finance
MmeBongnda Winifred B. Beriliy Director of Library
xvii
THE ADMINISTRATIVE AND TEACHING STAFF OF THE FACULTY OF
HEALTH SCIENCES (FHS), THE UNIVERSITY OF BAMENDA
2018/2019 Academic Year
1. Administrative staff
Prof Dora Mbanya Dean
Prof Christopher TangnyinPisoh Vice-Dean in charge of Academic
Affairs
Prof Helen KuokuoKimbi Vice-Dean in charge of Admissions
and
Records
Prof Henri Lucien F.Kamga Vice-Dean in charge of Research
and Cooperation
MrJacobTitafanVoma Faculty Officer
Dr Gerald Ngo Teke Chief of service,programmes,
Teaching
And research
Dr Moses Samje Chief of Service, Administration
and
Personnel
Mr Zaccheus Aweneg Chief of Service, Finance
Mr Humphrey Njoamomoh Chief of Service, Admissions and
Records
Mr Leonard Peyechu Chief of Service, Materials And
Maintenance
Dr Mary Garba Chief of Service, Internships
xviii
Mr Cyprien Bongwong Stores Accountant
2. Heads of Departments
Prof Frederic Agem kechia Biomedical Sciences
Prof Christopher Tangnyin Pisoh Clinical Sciences
Prof Bih Suh Mary Atanga Nursing/Midwifery
Dr Esther Etengeneng Agbor Medical Laboratory
Sciences
Teaching staff
a) Professors
1.
|
Christopher Kuaban
|
Internal Medicine/Chest Medicine
|
2.
|
Helen Kuokuo kimbi
|
Medical Parasitology
|
3.
|
|
Dora Mbanya
|
Haematology
|
|
b) AssociateProfessors
1.
|
BihSuh Mary Atanga
|
Nursing/Midwifery
|
2.
|
Henri Lucien F. Kamga
|
Medical Parasitology
|
3.
|
Frederic Agem Kechia
|
Medical Mycology
|
4.
|
|
Christopher Tangnyin Pisoh
|
General Surgery
|
|
c) Senior Lecturers
1.
|
Esther EtengenengAgbor
|
Nutritional Biochemistry
|
2.
|
Marie Ebob Bissong
|
Medical Microbiology
|
3.
|
Flore Ngoufo Nguemaim
|
Medical Parasitology
|
4.
|
Gerald Ngo Teke
|
Pharmacology
|
5.
|
|
Omarine Nfor Njimanted
|
Medical Parasitology
|
|
6.
xix
Moses Samje Biochemistry
7. William AkoTakang Obstetrics/Gynaecology
d) Assistant Lecturer
Jacob TitafanVoma Physics
e) Instructors
1. Kwende Odelia Nursing
2. Foba Marcelline Nursing
xx
HIPPOCRATIC OATH
« I solemnly pledge to dedicate my life to the service of
humanity;
The health and well being of my patient will be my first
consideration ;
I will respect the automomy and dignity of my patient ; I will
maintain the utmost respect for human life ;
I will not permit considerations of age , disease or
disability , creed , ethnic origin , gender , nationality , political
affiliation , race , sexual orientation , social standing , or any factor to
intervene between my duty and my patient ;
I will respect the secrets that are confided in me , even
after the patient has died ;
I will practise my profession with conscience and dignity and
in accordance with good medical practice ;
I will foster the honour and noble traditions of the medical
profession ;
I will give to my teachers , colleagues , and students the
respect and gratitude that is their due ;
I will share my medical knowlegde for the benefit of the
patient and the advancement of health care ;
I will attend to my own health , well being ,and abilities in
order to provide care of the highest standard ;
I will not use my medical knowledge to violate human rights
and civil liberties, even under threat ;
I make these pomises solemnly , freely , and upon my honour.
»
1
CHAPITRE ONE
INTRODUCTION
I.1) BACKGROUND
Bacterial meningitis is an infectious disease characterized by
infection and inflammation of the meninges due to the penetration and
multiplication of the bacterium in the cerebrospinal fluid. It results in
significant morbidity and mortality globally [1][2], and is
estimated to be fatal in 50% of cases with affecting approximately 1.2 million
people each year with two thirds occurring under 5 years of age
[2].
In USA, bacterial meningitis was responsible for an estimated
4100 cases and 500 deaths annually between 2003 and 2007, while in 2012, in
Africa World Health Organization identified 22000 meningitis cases in 14
countries in the meningitis belt [1][ 3].
Meningitis can be difficult to diagnose clinically
particularly in young infants who do not seem to reliably display the classic
features of the disease [4], where symptoms observed vary from
the bulging fontanel in neonates to frank meningeal signs in older children,
thus high index of suspicion is needed [5].Sequelae vary based
primarily on the etiologic agent [6] where higher mortality
rates tend to be associated with Haemophilus influenza type b
meningitis, pneumococcal meningitis and meningococcal meningitis
[7].
The different etiologies of bacterial meningitis in children
were observed in various studies where Anouk et al demonstrated in 2018 that in
Northern America Streptococcus pneumoniae was the most common pathogen
with weighted mean 43.1% [1].
Touré et al also showed in 2017 in Ivory Coast in a
study that Streptococcus pneumoniae and Neisseria meningitidis
were the commonest incriminated pathogens [8]. In
Cameroon in a study in 2014, it was seen that the incidence of bacterial
meningitis still remained high despite the introduction of vaccins against the
three most incriminated bacteria, notably Haemophilus influenzae which
was the most common pathogens constituting 39.2%, followed Streptococcus
pneumoniae with 31.6% and Neisseria meningitidis 10.5%
[3].
2
I.2) RATIONALE AND JUSTIFICATION
Bacterial meningitis is a serious often disabling and fatal
infection which causes 170,000 deaths worldwide each year [5].
On a review on meningococcal meningitis particularly, it was
demonstrated that the rate of 15 cases per 100,000 per week for two weeks
provokes vaccination of children aged greater than 2 years with one injection
of group A and C polysaccharide. Even still only about 50 % of cases of
meningitis are preventable[9].
Despite the development of vaccines, ad useful tools of rapid
identification of pathogens and potential antibiotherapy, bacterial meningitis
still remains a significant cause of preventable childhood deaths and a major
cause of neurological deficits and physical handicaps in children [5][
10], especially in sub-Saharan Africa where populations seem to be
more exposed to the different causative agents than any other part of the
world.
Though many reviews, above 100, have been conducted on
bacterial meningitis, especially in Cameroon, we can agree from those reviews
that this disease still makes one to ask question on the control and prevention
of the disease, through the implementation and correct dispensing of the three
main vaccins against the three major causative agents which are;Haemophilus
influenzae typeb ,Neisseria meningitidis and Streptococcus
pneumoniae.
Therefore, the purpose of this study is in line with the goal
3.2 of Sustainable Development Goal (SDG) which has as objective to ensure by
2030 a decrease, of avoidable death of newborns and children of less than 5
years [11], to determine the new incidence, etiologic agents,
clinical manifestations and hospital outcome of bacterial meningitis in
children.
I.3 ) MAIN OBJECTIVES
To identify the common pathogens responsible for bacterial
meningitis and to describe the hospital outcome in children with bacterial
meningitis.
I .3.1) SPECIFIC OBJECTIVES
1) To determine the incidence of bacterial meningitis in
children in the pediatric unit at the YGOPH.
2) To identify the common pathogens responsible for bacterial
meningitis in children at YGOPH.
3) To describe the different clinical manifestations of
bacterial meningitis in children at YGOPH and the evolution.
4) To describe the hospital outcome in children with bacterial
meningitis I.5) RESEARCH QUESTION
1) What are the incidence, etiologies, clinical presentation and
hospital outcome of bacterial meningitis in children at the YGOPH?
I.6 CONCEPTUAL FRAMEWORK

Sociodemograp hic
Characteristics
- Age
- Gender
- Vaccinati
on status - Immune
status
BACTERIAL VIRAL
ETIOLOGIES
-S. pneumoniae
-N.meningitidis
-H.influenzae
CHILDHOOD MENINGITIS
CLINICAL PRESENTATION
TREATMENT
HOSPITAL OUTCOME
? Mortalit y
? Cure
? Disabilit y
3
4
CHAPTER TWO
LITERATURE REVIEW
II.1) GENERAL OVERVIEW
The World Health Organization (WHO) recognizes Neisseria
meningitidis, in most countries to be the leading cause of meningitis and
fulminant septicemia and is also recognized to be a significant public health
problem [12] However, large recurring epidemics affect an
extensive region of sub-Saharan Africa known as the « THE MENINGITIS BELT
» (Figure 1) which comprises of 26 countries from Senegal in the West to
Ethiopia in the East [13].

Figure 1: Meningitis Belt in West Africa [14]
Most meningitis cases and out breaks in the African meningitis
belt occur during the epidemic season which tend to extend from November to
June depending on the region [13], with sub-Saharan region
having the world's greatest disease burdens of Haemophilus influenzae
type b streptococcus pneumoniae, and Neisseria meningitides
[15]. An enhanced meningitis surveillance regional
network is also available where the 23 countries participating (Figure 2)
reported in 2017 a total of 29827 of suspected cases of meningitis including
2276 deaths [13]. This was said to represent an increased
5
number of cases compared with 2016 of 18178 suspected cases
resulting also in an increased number of epidemic districts from 42 in 2016 to
57 in 2017[13]

Figure 2: Countries trained to conduct surveillance
for the Pediatric Bacterial Meningitis Surveillance Network, by performance
level* --- World Health Organization African Region, 2008 [16]
6
II.2) ETIOLOGIES AND RISK FACTORS OF BACTERIAL
MENINGITIS IN CHILDREN
In 2000, Hib and S. pneumoniae infections are
accountered for approximately 500,000 deaths in the sub-Saharan region.N.
meningitidis has been responsible for recurring epidemics resulting in
700,000 cases of meningitis [17]

Figure 3: Causes of confirmed bacterial meningitis
from eleven years of active surveillance in a Mexican hospital, 2005 -2016.
[18]
? STREPTOCOCCUS PNEUMONIAE
Streptococcus pneumoniae is one of the main causing
agents responsible for meningitis in newborns, in young children and teenagers
with higher rates of lethality and morbidity [19] [20].
Streptococcus pneumoniae is a Gram - positive, encapsulated bacterium
often found as a normal commensal in the nasopharynx of healthy children
[20].Streptococcus pneumoniae was the commonest cause of
bacterial meningitis in US and Europe, and tends to occur mostly among children
older than 5 years of age [10]. However, the highest risk of
bacterial meningitis caused by Streptococcus pneumoniae
7
is in children greater than 2 years [21]. The
bacterium can become pathogenic, with invasive disease, greatest in patients
who develop meningitis.
? VIRULENCE FACTOR AND PATHOGENESIS
The bacterium is spread by the respiratory fluids from the
infected person when they cough or sneeze, the bacterium then finds its way in
the system where it escapes to the local host defenses and phagocytic
mechanisms, then penetrates the CSF either through choroid plexus /
subarachnoid space originating from bacteremia or via direct extension from
local respiratory system infections [20]. It is able to escape
into the central nervous system easily with the aid of pneumococcal proteins
which include [22]:
> Pneumococcal surface protein A (PspA)
:It is located in the cell wall of the bacterium and acts as a
protective antigen against the host complement system.[22]
> Hyaluronate lyase (Hyl) :This enzyme
mediates facilitation of tissue invasion by breaking down the extracellular
matrix component of the host cell ,thereby increasing tissue permeability.This
factor aids in the pathogenesis of wound infection, meningitis and even
pneumonia[22].
> Autolysin (LytA) :These enzymes are
located in the cell envelope and has a very important role in cell wall
degradation which leads to cell death .They degrade the peptidoglycan backbone
of bacterial organisms , which leads to cell lysis[22].
> Pneumococcal surface antigen A (PsaA) :
This protein is thought to have protective properties and is anchored
to S.pneumoniae through bacterial cell membrane[22].
> Choline binding protein A (CbpA) : It
serves as an anchoring device to pneumococci lipoteichoic C acid structures
present on the surface of the bacterium.Thus aids in the adherence and host
tissue colonization[22].
> Neuraminidase: They enhance colonization
due to their action on gylcans where , they cleave terminal sialic acid from
cell surface gylcans such as mucin, glycolipids and glycoproteins which is
probably responsible for damage to host cell gylcan[22].
Children with basilar skull fractures with CSF leak, asplenism
and HIV infection are at particular risk of developing pneumococcal meningitis
[23], pneumococcal conjugate vaccines have been implemented in
many countries, and immunization with the heptavalent pneumococcal vaccins PCV7
has decreased incidence by incriminating
8
pathogen by greater than 90% [24]. Meanwhile
pneumococcal population undergoes temporal changes in clonal distributions in
the absence of pressure from a vaccine [24].
? HAEMOPHILUS INFLUENZAE
Haemophilus influenzae is Gram-negative coccobacilli
capable of causing serious invasive disease in the child of less than 5 years
of age (Figure 3). Haemophilus influenzae encapsulated serotypes are:
a, b, c, d, e, and f which facilitates its penetration in the blood with the
serotype b being the most virulent of all. The pathogen does not stay alive for
a long time in the environment, it thus has a 12 hours' survival on plastic
objects [25].Haemophilus influenzae type b was the
most common cause of life threatening infection in children in industrialized
countries until universal immunization, where children of less than 5 year of
age, were the primary host with 39% of nasopharynx colonization, but nowadays,
it is instead older children and adults that are considered to be more
susceptible carriers shifting them to primary host[26][25].
? VIRULENCE FACTOR AND PATHOGENESIS
The transmission of the pathogen is done through droplets from
the respiratory airways, through cough, sneezing, speaking from colonized
person, through saliva, and contaminated objects from respiratory secretions.
Sodium hypochlorite at 1%, ethanol at 70%, formaldehyde, glutaraldehyde has
good efficacy against Haemophilus influenzae
[25].However Hib , though not known to produce toxins, it
has the capacity to invade the host system using the following defense
methods[27] :
? Polysaccharide capsule : It is a very
important virulence factor of encapsulated strains of Haemophilus influenza
strains and it protects the bacterium from host immune functions[27].
? Lipooligosaccaharide (LOS) : A major
component of the outer leaflet of the Gram -negative bacteria outer membrane ,
which mediates interactions between bacteria and the host immune system[27].
Hib apart from using the above defense mechanism also uses ,
particular processes to escape from complement systems such as;
- Phase variation which is a immune evasion strategy during
infection where the outer surface of the bacterium is modified to adapt to
changes in the host environment[27].
9
- Binding of host complement regulatory factors which is
important during colonization and infaction, where these factors block activity
at various step of the complement pathway[27].
Globally, Hib accounts for approxi mately 8-13 million serious
illness annually, including 173.000 cases of meningitis causing 78.000 deaths
[28]. The incidence of bacterial meningitis due to the
pathogen has been experiencing a drop in its incidence in developed
countries[8], in Belgium it was at 0,04/100,000 inhabitants in
2012 and even in developing countries where there is implementation of the
vaccin against the Haemophilus influenza type b, less prevalence was
noticed compare to previous years[25]. Despite its reduction
in the cause of meningitis, its identification and prompt treatment are
essential because of the short incubation period which is 2 - 4 days
[25].World Health Organization recommended the addition of Hib
vaccine to immunization programs , according to national capacities and
priorities, however, uptake in developing countries has remained
slow[26].This is partly due to the uncertainty about the true
disease burden[26].
? NEISSERIA MENINGITIDIS
Meningococcal infections occur worldwide as endemic
disease(Figure 3)[29], and it appears that the occurrence of
invasive meningococcal disease is not solely determined by the introduction of
a new virulent bacterial strain but also by other risk factors determining the
transmission of the pathogens [29][19]. Meningococcal
meningitis occurs when Neisseria meningitidis multiplies on the
meninges and in the CSF [30]. Early recognition of this type
of meningitis is important than in any of the acute infectious diseases
[31].
Neisseria meningitidis is a Gram -negative diplococci
which has 13 serogroups defined by specific polysaccharide designated A, B,C,H,
I, K, L, M,X,Y,Z, 29E, and W135(serogroup D is no longer recognized),but is A,
B,C, W135, X, and Y account for most disease where group A is mostly found in
Sub-Saharan Africa, group B found in the temperate climates and group C occurs
mostly as outbreaks [29][32].
Neisseria meningitidis is found in the oropharynx of
10 % of the population with an annual number of invasive disease cases
worldwide estimated to be atleast 1,2 million with 135,000 deaths related to
invasive meningococcal disease and WHO categorizes countries by risk of
meningococcal disease as follows [32];
10
? High risk: countries with greater than 10 cases /100,000 and
/or =1 epidemic Over last 20 years
? Moderate risk: Countries with 2-10 cases /100,000 population
per year
? Low risk: Countries with less than 2 cases /100.000 populations
per year.
The proportion of cases caused by each serogroup varies by age
group also geographic distribution and epidemic potential differ according to
serogroup.Neisseria meningitidis ends to be present particularly in
children less than 5 years old with estimated 500,000 cases and 50,000 deaths
globally each year [29].The largest burden of meningococcal
disease occurs in the sub-Saharan Africa during dry season with the presence of
dust , winds , cold nights with the upper respiratory tract infections combine
to damage the nasopharyngeal mucosa increasing the risk of the disease which is
transmitted through droplets of respiratory secretions while Invasive disease
developing in a small percentage of carriers is regarded as
emergency[32][33].
? VIRULENCE FACTOR AND PATHOGENESIS
Neisseria meningitidis is a fastidious, encapsulated
aerobic bacterium that colonises host mucosal surface using multiple factors
such as[34] :
> Capsule : It is present in strains that
cause invasive disease ,since it provides resistance to antibody and complement
-mediated killing and inhibits phagocytosis[34].
> Lipolysaccharide (LPS) : Induces the
release of chemokines , reactive oxygen speies and nitric oxide and has a role
in resistnce to other host defense[34].
> Adhesins pili : Initiate binding to
epithelial cells,and facilitate passage through the epithelial mucus layer and
movement over the epithelial surfaces .They also facilitates the uptake of DNA
by meningococci and enable adherence to endothelial cells and
erythrocytes[34].
> Opacity proteins : Opa and Opc (only
expressed in Neisseria meningitidis) while Opa is expressed by both
meningococci and gonococci.They have potential roles in pathogenesis that is
not well understood[34].
> Porins : Por A and Por B are porins
through which small nutrients diffuse to the bacterium and they are also
involved in host cell interactions and they are targets for bactericidal
antibodies.Por A is the main component of vesicle based vaccines and a target
for bactericidal antibodies while Por B insert in membranes and induce Ca 2+
influx and activates TLR2 causing cell death[34].
11
? Iron binding proteins: They enable the
meningococci to acquire iron which is an important growth factor during
colonization and disease[34].
World's Health Organization policy's of epidemic containments
prevents at best 50% of cases, therefore for an effective prevention of
meningococcal meningitis in sub Saharan Africa, there should be a strict and
effective follow up of universal vaccination recommendation, but still more
than half of cases among infants less than 1 year are caused by serogroup B
meningococci for which no vaccins is available. Also serogroup X, previously a
rare cause of sporadic meningitis, has been responsible for outbreaks between
2006 and 2010 in Kenya, Niger, Togo, Uganda, and Burkina Faso, the latter with
1,300 cases among the 6, 732 reported annual cases [9][
32].
II.3) RISK FACTORS ASSOCIATED WITH THE OCCURENCE OF
BACTERIAL MENINGITIS IN CHILDREN
The human infection with meningitis has seasonal variation and
this differs from one country to another [33]. Worldwide
meningitis was estimated to cause 1.73.000 deaths in 2002, most children from
the developing countries [35]. Bacterial meningitis as any
other disease has factors that may be associated to its development, and they
can be preventable or not as follows;
? AGE: The first age group (less than 1year)
occupies the highest number of incidence of the disease which tends to be
higher in developing countries than developed countries. The cause might be due
to the immaturity of immune system, lack in the pre-exposure of the body to the
most incriminated organisms which enhances the memory of the immune system to
fight against the invaders[33].
? GEOGRAPHIC ZONE AND CLIMATE: Bacterial
meningitis is endemic in the sub-Saharan region of Africa, especially in those
countries that are included in the «Meningitis Belt» which is made up
of 26 countries from the Senegal to the West to Ethiopia to the East.
Meningitis in tropical areas occurs in dry season and decrease in periods of
rains, while in temperate regions, the epidemics usually occur during winter
and spring seasons [13][ 33].
? SEX: The male sex has been observed in
various studies to be a risk factor for bacterial meningitis. It is not yet
well understood why males will be more susceptible to getting the disease than
female sex [33].
12
? LOW SOCIOECONOMIC STATE AND CROWDING LIVING
CONDITIONS: These are factors that are mostly seen in developing
countries.Crowdness encourage development of meningitis since most of the
detected pathogens are air transmissible [33][35].
? PASSIVE SMOKING: Children exposed to
smoking are found to get meningitis because, passive smokers tends to harbor a
greater number of bacteria in their throat and nasal passage. Also smoking
plays an important role in diminishing the capacity of epithelial cells
covering the respiratory tract for prevention of acquiring infection in
addition to the prevalence of healthy carrier of pathogens [33][
35].
? RECENT UPPER RESPIRATORY TRACT INFECTION:
This can easily be explained by the route of entrance of the
microorganisms to the brain and those important routes of infection are: Otitis
media, mastoiditis, sinusitis and pneumoniae [33].
? HISTORY OF HEAD INJURY AND BRAIN SURGERY:
It is considered an important risk for development of bacterial
meningitis, because of the proximity of the injury with the central nervous
system [33].
? MALNUTRITION: Malnutrition is a complex
disease that if not well controlled affects every system of the body including
the hematopoietic system, and most of the time complicates with anemia. Anemic
patients are highly susceptible to serious infections such as bacterial
meningitis and can be caused by different etiologies [35]. OTHER
FACTORS ASSOCIATED WITH THE DEVELOPMENT OF BACTERIAL MENINGITIS:
? Bottle feeding[33]
? Compromised immune system[33]
? Splenectomy[33]
? Sickle cell disease[33]
? Inherited family tendency for
meningitis[33]
II.4) PATHOPHYSIOLOGY OF BACTERIAL MENINGITIS
There are conditions required to cause invasive diseases such as :
II.4.1) BACTERIAL INVASION
Bacteria reach the central nervous system either by
hematogenous spread or by contiguity like in the case of neonates and children
where pathogens are acquired from
13
non-sterile maternal genital secretions and from organisms
that colonize the upper respiratory tract respectively[29]
Successful colonization of the nasopharyngeal mucosa depends
on the ability of bacteria to evade host defenses including secretory Ig A and
ciliary clearance mechanisms, and to adhere to mucosal epithelium[29].
Microbial virulence factors include the Ig A protease secreted by Neisseria
meningitidis, Streptococcus pneumoniae and Haemophilus influenzae that
cleave Ig A to an active form. Notably meningococus depends on the binding of
fimbriae on the bacterial cell surface to adhere on epithelial cells, and
non-encapsulated strains of meningococci adhere better than capsulated strains.
As the mucosa has been breached and the intravascular space has been entered,
the pathogen must survive in the circulation in order to penetrate the blood
brain barrier[36]. The principal host defense mechanism is complement although
neutrophil and antibodies are also important(Figure 4). The meningeal pathogens
are all capsulated and this virulence factor of theirs enables them to evade
phagocytosis and bactericidal activity of the complement system. In
Streptococcus pneumoniae infection, the alternative complement pathway
is activated by pneumococcal capsular polysaccharides, where there is direct
cleavage of the C3 which generates C3b which opsonizes the organism, enhancing
phagocytic clearance from the circulation[37]. The C3b then binds to Factor B
on the pneumococcal capsular surface offering resistance to opsonisation.
Therefore, it is understandable why individuals with impaired complement
systems are at high risk of getting all the manifestations of invasive
pneumococcal disease.
Neisseria meningitidis, has its capsular sialic acids
which facilitates binding to the C3b to the complement regulatory protein
Factor H, thus blocking activation of the alternative pathway by presenting the
binding of C3b to factor B[36].
In order to cross the blood brain barrier and to overcome
structures such as tight junctions, meningeal pathogens carry effective
molecular tools. They cross the blood brain barrier to enter the subarachnoid
space and are aided with the presence of specific surface bacterial proteins
like E. coli proteins IbeA,IbeB and ompA, Streptococcal proteins such
as CbpA which interacts with glycoconjugate receptor of phosphorlcholine with
platelet activating factor (PAF) on the eukaryotic cells and promotes
endocytosis and crossing the blood brain barrier.N. meningitidis
proteins Opc,
14
Opa, PilC, and a Pili protein[36]. Bacteria causing meningitis
in newborns, most importantly group B streptococcal and Escherichia coli are
also well equipped with adhesive proteins allowing them to invade the central
nervous system[37].
II.4.2) INFLAMMATORY RESPONSE
The lack of host defenses in the CSF allows rapid
multiplication of bacterial pathogens resulting in the release of microbial
products such as lipopolysaccharide[36]. The hall mark of bacterial meningitis
is recruitments of neutrophils into the cerebo spinal fluid(Figure 4)[37].
Neutrophil extravasation to any site of inflammation depends on the coordinated
sequential expression at the cell surface of specific adhesion molecule,
notably L-selectin (CD62l) is expressed at the cell surface and allows the
neutrophil to «roll» along the endothelium.For extravasation to
proceed L-selectin must be removed from the surface of neutrophil and
expression of the B2 integrin CD11b /CD18 must be upregulated[36].
Neutrophil adherence to endothelium occurs through the
interaction of neutrophil CD11b/CD18 and diapedesis and migration of neutrophil
along a chemotactic gradient to focus of inflammation that follows. The removal
of L-selectin and integrin upregulation are achieved by neutrophil activation
which occurs when the cell encounters activated endothelium (IL-8 and PAF are
typical activators of endothelium)[36].
II.4.3) RAISED INTRACRANIAL PRESSURE
Intracranial pressure often rises in meningitis and can lead
to life threatening cerebral herniation. Three pathophysiologic mechanisms
contribute to the development of cerebral oedema[37]. They are;Vasogenic,
Cytotoxic and Interstitialoedema. Vasogenicoedema occurs directly as a result
of the increased permeability of the blood brain barrier[37]. Cytotoxic oedema
is the rise in intracellular water due to loss of cellular homeostatic
mechanism and cell membranes function, attributed to the release of toxins from
neutrophil or organisms. Anti-diuretic hormone (ADH) release leads to
hypotonicity of cerebral extracellular fluid and increase the permeability of
the brain to water. Interstitial oedema is the result of an imbalance between
cerebo spinal production and resorption, and occurs when blood flow or cerebo
spinal resorption is impaired[37].

15
Figure 4 : Pathogenesis of bacterial meningitis
[38]
II.4.4) NEURONAL DAMAGE
Bacterial meninges causes disabling neuropsychological
deficits in up to 50 % of its survivors with the hippocampus most affected and
vulnerable area of the brain. The extracellular fluid around the brain cell is
contiguous with the cerebo spinal fluid and the proximity to the ventricular
system allows diffusion between those compartments around could deliver soluble
bacterial and inflammatory toxic mediators[37].
16
Neuronal damage in meninges involve bacterial toxins,
cytotoxic products of immune competent cells and indirect pathology secondary
to intracranial complications(Figure 4)[38]. In the case of Streptococcus
pneumoniae is associated with the highest frequency of neuronal damage, produce
two major toxins identified; H2O2 and Pneumolysin a pore forming cytolysin[37].
They cause programmed death of neurons and microglia by inducing rapid
mitochondrial damage. Pneumolysin translocate to mitochondria and induce pore
formation in mitochondrial membranes. Release of apoptosis inducing factor
(AIF) from damaged mitochondria leads to fragmentation of DNA and apoptosis
like cell death[37]. The cell death is executed in the Caspase-independent
manner, where cells exposed to live Pneumolysin cannot be rescued by caspase
inhibitors but somehow any intervention from those inhibitors, z-VAD-fmk there
is a 50% chance to avoid neuronal damage[37].
II.5) DIAGNOSIS OF MENINGITIS IN CHILDREN
II.5.1) CLINICAL DIAGNOSIS OF MENINGITIS IN
CHILDREN
The clinical symptoms and signs of bacterial meningitis in
children vary depending on the age of the child and duration of disease. The
classic symptoms of meningitis are fever, headache, photophobia and neck
stiffness. However, in the early stages of meningitis, and particularly in
young children, the symptoms of meningitis can be variable or nonspecific and
the classic symptoms may be absent, making meningitis difficult to diagnose.
Nonspecific signs include abnormal vital signs such as tachycardia and fever,
poor feeding, irritability, lethargy, and vomiting [23][
39].
Children may have fever and vomiting associated with
irritability, drowsiness and confusion. They may become suddenly ill with fever
and rigors, which can be mistaken for seizures. Also muscle and joint aches can
occur which can be responsible for children being restless and miserable.
Vomiting, nausea and poor appetite are common while abdominal pain and diarrhea
are less common. Meningitis causes a rise in intracranial pressure [39
]. It presents in babies and in young children as a bulging or full
fontanel. In children without an open fontanel, raised intracranial pressure is
seen as other features like systemic hypertension with bradycardia. Children
may have an abnormal tone, jerky movements or be floppy
[39][40].
Other children are more likely to have the classic features of
meningitis, fever, vomiting and headache, stiff neck and photophobia.
17
Rashes may be present, most commonly when the causative
organism is Neisseria meningitidis, but more likely to be absent,
atypical, scarce or petechial in character than those seen in meningococcal
septicemia [41].
II.5.2) PARACLINICAL DIAGNOSIS OF BACTERIAL MENINGITIS
II.5.2.1) LUMBAR PUCTURE AND CSF ANALYSIS
A lumbar puncture and CSF analysis is the gold standard and
definitive diagnosis of bacterial meningitis. It is done in either sitting or
lateral decubitus position and is important to monitor the patient visually and
with a pulse oximetry for any signs of respiratory difficulties as a result of
the assumed position.
The subarachnoid space should be entered below the level of
spinal cord termination (Figure 5), and any of the interspace between L3 -L4
and L5 -S1 in children
Analysis of CSF should include: Gram stain and cultures; white
blood cell (WBC) count and differential; Glucose and protein concentrations;
Cytocentrifugation of the CSF enhancing the ability to detect bacteria and
perform a more accurate determination of the WBC differential
[23].
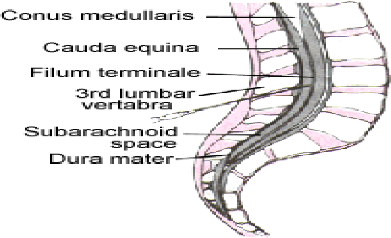
Figure 5: Lumbar spine anatomy[41].
18
Typically, the CSF white cell count (wcc) is >1000
cells/mm3 although it may not be elevated in the early phase of the infection
and the majority of white cells are polymorph nuclear (PMNs). CSF protein is
typically elevated (100-200 mg/dL) and glucose low (CSF to serum ratio <0.4)
[23]
A reduced absolute CSF concentration of glucose is as
sensitive as the CSF-to serum glucose ratio in the diagnosis of bacterial
meningitis [21]
The Gram-stained smear of CSF has a lower limit of detection
of about 105 colony-forming units/mL. Of patients with untreated bacterial
meningitis, 80% to 90% have a positive CSF Gram stain. Unless unusual
pathogens, such as anaerobes, are suspected, agar plate cultures of CSF are
preferred to liquid media [21][ 23].Pleocytosis is a typical
finding in bacterial meningitis, the WBC count usually greater than 1000
cells/mm3, and there is a predominance of polymorphornuclear leukocytes.
The lumbar puncture for the cerebro spinal analysis should be
performed once the diagnosis of meningitis is suspected and after the patient
is stabilised. However, there can be reasons to delay lumbar puncture which
include the following
+ Local site for lumbar puncture: Skin infection at site of
lumbar puncture, and
anatomical abnormality at the site of lumbar puncture
site[42]
+ Patient instability: Respiratory or cardiovascular compromise,
and continuing
seizure activity[42]
+ Suspicion of space occupying lesion [42]
+ Raised intracranial pressure[42]
+ Focal seizures[42]
+ Focal neurological signs[42]
+ Reduced conscious state of GCS less than 8 and especially if
patient is comatose[42]
+ Decerebrate or decorticate posturing[42]
+ Fixed dilated or unequal pupils[42]
+ Absent dolls eye movement[42]
+ Papilledema [42]
+ Hypertension or bradycardia[42]
+ Irregular respirations[42]
+ Anticoagulations and bleeding disorders[42]
19
II.5.2.2) COMPLICATIONS OF LUMBAR PUNCTURE
As in any other procedure, there might be complications after,
and in lumbar puncture some complications have being observed such as;
1. Postural puncture headache (PDPH): It is usually
self-limiting, but when serious, supportive treatment for the PDPH and its
accompanying symptoms include: bed rest, analgesics, hydration,
corticosteroids, and anti-emetic medications [43]. Also in
case of severe and persistent headaches, injecting saline into the epidural
space may be therapeutic [43].
2. Local back pain [43]
3. Infection[43]
4. Spinal hematoma[43]
5. Subarachnoid epidermal cyst[43]
6. Apnea[43]
7. Herniation (post procedural cerebral herniation):
Herniation can be the direct cause of death in around 30% of such children.
Therefore, it is practical to perform cranial CT imaging to evaluate any such
abnormalities before performing an LP [41][ 43].
8. Transient limp or paresthesia[43]
9. Transient ocular palsy[43]
10. Cerebral Herniation[43]
II.5.3) OTHER LABORATORY INVESTIGATIONS
Despite the fact that CSF analysis from lumbar
puncture is the gold standard in the diagnosis of meningitis , some other tests
could be performed like the following:
? C-REACTIVE PROTEIN (CRP) :
Clinically it is not easy to differentiate between bacterial and viral
etiologies in patients with suspected meningitis, and due to the high mortality
rate and potential neurological sequelae in survivors, there is an urgent need
for rapid diagnosis with a near 100 % sensitivity. CRP was used and is still
used as the biomarker for inflammation and tends to be elevated in both viral
and bacterial infections, limiting its ability to discriminate between
bacterial and viral etiologies of meningitis [44].
20
? PROCALCITONIN :Procalcitonin (PCT) is now
considered to be the best candidate to replace CRP due to its high diagnostic
accuracy in various infectious pathologies, including sepsis, acute infections,
endocarditis, and pancreatitis. Normal PCT levels of healthy individuals are
less than 0.1ng/ml and level increase drastically in response to bacterial
infections, unlike CRP, PCT has not been reported to the elevated in viral
infections, thus conferring it to the important ability to distinguish easily
between bacterial and viral etiologies [44].
- PCT also shows utility in the early diagnosis of meningitis
by rising after 4h, peaking at 6h and remaining elevated over 24 h. This is in
contrast to CRP, which rises over 6-12h and peaks at 24 -48 h. This delay in
diagnosis combined with the traditional 72h wait for results of Gram stains,
often results in patients receiving empiric antibiotics
[44].
? Full blood count, Serum electrolytes and Coagulation
studies: Normally these investigations are required initially before
thinking about lumbar puncture, in order to assess for sepsis complications
[21].
? Serum Glucose: This test must be measured
routinely in a child having meningitis, since in a state of hypoglycaemia (low
glucose in blood) seizure might occur and it can be the cause of convulsion in
children apart from the presence of uncontrolled fever
[21].
? Blood Cultures: Cultures are also important
in the diagnosis of the disease especially in those patients having
contraindications towards lumbar puncture [21].
? Normally all the above usually changes in a
declining pattern after the introduction of antibiotics , but some authors
propose Latex agglutination , as a reliable test to detect bacterial capsular
antigens in patients with suspected bacterial meningitis and have been
receiving antibiotics all the time lumbar puncture was performed and it is also
estimated that in the future a more sensitive technique like 16rRNA gene by
polymerase chain reaction might help in the diagnosis of bacterial meningitis
in patients already on antibiotics[23].
21
II.5.3.1) IMAGING TECHNIQUES
? COMPUTED TOPOGRAPHIC SCAN (CT SCAN): This
should normally be done before lumbar puncture in order to rule out any
presence of raised intracranial pressure in order to avoid brain herniation
during a lumbar puncture performance. Now it should be noted that, CT SCAN is
not sensitive at 100%, because a normal SCAN does not absolutely exclude
subsequent risk of herniation[21].
? MAGNETIC RESONANCE IMAGING (MRI): It is
also useful in the diagnosis meningitis and is more sensitive than the SCAN
with the fact that, it is able to show extensive inflammatory tissue
destruction of the meninges in their different spaces, also detects pus and
thus can be used when the others are not available or feasible[45].
II.6) CURRENT TREATMENT ON BACTERIAL MENINGITIS IN
CHILDREN
The three major aspects of treatment of bacterial meningitis
include (1) antibiotic therapy (2) fluid restriction (3) adjunctive therapy
[21].
II.6.1) ANTIBIOTIC TREATMENT
Most treatment guidelines recommend the use of a
third-generation cephalosporin (such as ceftriaxone or cefotaxime) in
conjunction with vancomycin as initial antibiotic therapy. Cefotaxime and
ceftriaxone have excellent activity against all Hib and N.
meningitidis strains [46].
Increasing resistance of S. pneumoniae to penicillins has been
reported, and although cefotaxime and ceftriaxone remain active against many
penicillin-resistant pneumococcal strains, treatment failure has been reported,
hence the addition of empirical vancomycin [46].
In resource limited settings the treatment of paediatric BM
generally has two protocols based on age (under 2 months and above 2 months of
age).Accordingly, for neonates and young infants (under 2 months of age) the
first-line antibiotics are Ampicillin and Gentamicin and alternatives, a
third-generation cephalosporin, such as Ceftriaxone or Cefotaxime plus
Gentamycin.For infants and children (above 2 months of age) the first line is
the combination of Penicillin G and Chloramphenicol and the alternative is
Ceftriaxone, or Cefotaxime [2].
22
II.6.2) ADJUVANT THERAPY AND SUPPORTIVE THERAPY
Recommended dexamethasone dosing regimens range from 0.6 to
0.8 mg/kg daily in two or three divided doses for 2 days to 1 mg/kg in four
divided doses for 2 to 4 days [47][48].
For optimal results, the first dose of dexamethasone could be
administered before, but due to its side effects in children like duodenal
perforation, it is better to administered it,concomitant with the first
parenteral antibiotic dose, since in either way the efficacy of the
corticosteroid still remains the same.
Control and prevention of seizures can be attained with
anticonvulsant medications; benzodiazepines, phenytoin, and phenobarbital are
commonly used for this purpose [21].
II.6.3) FLUID RESTRICTION
In general, it is a common practice to restrict fluids to two
thirds or three quarters of the daily maintenance during the management of
childhood meningitis. The basis for this practice is the need to reduce the
likelihood of the syndrome of inappropriate secretion of antidiuretic hormone
(SIADH). SIADH is characterized by hyponatraemia, fluid retention and a
tendency to worsen cerebral oedema in meningitis. Therefore, practitioners
reduce fluid therapy in children with meningitis in the hope of preventing
SIADH [21].
II.7 PREVENTION OF BACTERIAL MENINGITIS IN CHILDREN
II.7.1) VACCINATION
Vaccination is the immunisation of someone against an
infectious disease through the administration of a vaccine. These vaccins act
by stimulating the immune system, thereby protecting from infection and / or
disease (WHO)[49]. Bacterial meningitis even though still an aggressive
infection, is preventable with the use of vaccines against its different
etiologies introduced and served mostly in children with less than 2 years of
age. This is because these children are more susceptible to infection with
encapsulated bacteria because of their immature immune system to respond
against the bacterium polysaccharide antigens[50].
23
Approximately 3/4 of deaths due to meningitis are prevented
with Hib and pneumococcal conjugate vaccines,which reduce nasopharyngeal
carriage of these organisms in the host and induce immunity[51].
Pneumococcal vaccins are available in two forms [52]:
Pneumococcal conjugate vaccine which is served in children
with less than 2 years of age and protects them against severe forms of
pneumococcal disease like; pneumoniae, meningitis and bacteremia.Two conjugates
are used PCV 13 with 13 serotypes and PCV 10 with 10 serotypes which are
relatively well tolerated. WHO recommends three primary doses starting as early
as 6 weeks of age or as an alternative, two primary doses could be given at the
age of 6 months plus a booster dose at 9- 15 months of age[53].
Pneumococcal polysaccharide vaccine which is served in adults
of greater than or equal to 65 years of age[53].
The different meningococcal polysaccharide vaccines include:
Bivalent(A and C)
Trivalent (A,C and W135)
Tetravalent (A,C,Y and W135)[54]
The Group A and C vaccines have a short term immunisation
effects in older children and adults and it should be noted that group C only
does not prevent disease in children with less than 2 years of age. Meanwhile
polysaccharide Y and W135 are efficient in children greater than 2 years of
age.Tetravalent vaccines are administered in single dose and in children as
from 1 year.These vaccines have as role to induce T cell 6 dependent immune
response and to reduce the nasopharyngeal carriage of meningococci[54].
The anti-Hib vaccine is mixed with a set of four other
vaccines (Pentavalent vaccine) which are vaccines against; diphtheria,
hepatitis B, tetanus and pertussis.Normally three doses are to be administered
for a good immunity, and the first dose is served as from 6 weeks. It can be
administered to 18 months maximum with atleast four weeks spacing in between
the doses[55].
24
II.7.2) CHEMOPROPHYLAXIS
Close contacts of all children with meningococcal meningitis
should receive chemoprophylaxis (ceftriaxone, rifampin, or ciprofloxacin), and
contacts of those with Hib should receive ceftriaxone or
rifampin[21].
Rifampin is administered 10 mg /Kg of body weight every 12
hours for children greater than or equal to 1 month of age, and 5 mg /Kg every
12 hours for infants less than 1 month of age. Rifampin is effective in the
eradication of nasopharyngeal carriage of Neisseria meningitidis. In
addition to rifampin, other antimicrobials are effective in the reduction of
nasopharyngeal carriage of meningococcal pathogens, like ciprofloxacin but
generally not recommended for persons less than 18 years of age because of its
destructive effect on cartilage. Whereas ceftriaxone administered in a single
dose of 125 mg in children is also effective [55].
Unvaccinated children less than 5 years of age should also be
vaccinated against H. influenzae as soon as possible. Patients should
be kept in respiratory isolation for at least the first 24 hours after
commencing antibiotic therapy [21].
II.7) COMPLICATIONS OF BACTERIAL MENINGITIS IN
CHILDREN
Meningococcal disease remains a major cause of morbidity and
mortality in childhood. Neurological disorders in children are common
occurrences in clinical practice. The disorder accounts for more than 170.000
deaths worldwide each year with majority of people affected living in Africa
[56][ 57].
Young children are particularly vulnerable to bacterial
meningitis, and when exposed poor outcomes may occur due to the immaturity of
their immune systems [57]. Two thirds of meningitis deaths in
low income countries occur among children less than 15 years of age. These
complications could be classified as [57]; short term, middle
term and long term.
SHORT TERM
- Brain edema is an early life threatening
complication, in which brain structure changes is not usually found in 80%, but
the residual stage of bacterial meningitis is characterised by cerebral
destructive /proliferative or atrophic changes of different severity
[58].
-
25
MID TERM
- Seizure could be placed in this term. Most
children present with recurrent seizure disorder.Seizures that occur early in
the course of bacterial meningitis are easily controlled and are rarely
associated with permanent or long term neurologic complications. In contrast,
seizures that are prolonged, difficult to control, or begin more than 72 hours
after hospitalization are more likely to be associated with neurologic
sequelae, suggesting that a cerebrovascular complication may have occurred
[57].
- Paresis: It is usually present but resolve
with time, which typically resolves from and intracranial abnormality suh as;
cortical vein, sagittal vein thrombosis, central artery spasm, subdural
effusion or empyema and cerebral infarct [57].
LONG TERM
The incidence , type and severity of sequelae is influenced by
infecting organisms, age of child and severity of acute illness , but it can be
difficult to predict with children.The potential impact of the illness is
further complicated by the fact that some of these sequelae may not become
apparent until months or years after the acute illness.These long term
complications include ;Visual loss ,Cognitive delay, Speech/language
disorders[,Behavioral problems,motor delay/impairment and attention deficit
hyperactivity[57].
- Hearing loss: It might be transient or
permanent. Transient hearing loss may be secondary to a conductive disturbance
affecting many patients. Permanent damage results from damage to the eighth
cranial nerves, bacterial invasion, cochlea or labyrinth induced by direct
bacterial invasion and /or inflammatory response elicited by the infection
[57].
26
II.8) PUBLICATIONS ON MENINGITIS OUT OF
AFRICA
· Almuneef et al in 1998 analysed bacterial etiologies
and outcome of childhood meningitis in Saudi Arabia and in his study there was
a predominance of female sex, also the most affected age being from 3 months of
age to 5 years. The presenting complaints in his study appearing in order of
decreasing frequency were; fever 86 %, vomiting 29 %, poor feeding 19 %,
seizure 14 % and lethargy 14 %[59]
· Franco -Paredes et al reviewed acute bacterial
meningitis cases in 2008, in Mexican patients aged from 1 month of age to 18
years, recorded the most affected group by bacterial meningitis to be between
1-6 months, with Hib being the common pathogen found in 50% of cases. Incidence
proven to have declined significantly after the introduction of appropriate
vaccins [60].
· Nicole Le Saux in 2014 reviewed the current
epidemiology of bacterial meningitis in children beyond the neonatal period in
Canada, and came to the conclusion that the incidence of bacterial meningitis
in infants and children has decrease since the routine use of conjugated
vaccines targeting Hib, Streptococcus pneumoniae, and Neisseria
meningitidis [61]
· Polkowska et al presented in Finland Streptococcus
pneumoniae and Neisseria meningitidis to be the most common pathogens with an
incidence drop from 1.88 to 0.70 in 2014[62].
· Incidence of bacterial meningitis dropped in Japan
from the records of Shingoh in 2015 of 1.19 in 2009-2010 to 0.37 in 2013 -2015,
confirming the efficacy of the Hib and PCV introduction
[63].
IN AFRICA
· In Niamey, in Niger in 1999, using a retrospective
surveillance on cases of laboratory diagnosed bacterial meningitis from
1981-1996 showed that the majority of cases were caused by Neisseria
meningitidisat 57 %, and there was a predominance of meningitis in males
occurring the dry season[64]
· Koko in Libreville, Gabon had predominance in the
female sex among the admissions of children with bacterial meningitis in 2000,
he also noted the highest mortality in children with less than 1 year of
age[65]
·
27
Mullan et al in 2011, evaluating records of cerebrospinal
fluid samples between 2000 to 2008 at Princess Marina hospital in Gabonone,
Botswana, reported Streptococcuspneumoniae(n=125) and
Haemophilusinfluenzae(n=60) to be the most common bacteria cultured,
present in less than or equal to 12 years old and less than 5 years
respectively. The author also reported the climatic tendency of the pathogens,
where Haemophilus influenza was mostly common between April and
September, while Streptococcus pneumoniae most common between May and
October[66]
· In Nigeria , Frank - Briggs in a study followed
patients post meningitis to assess outcome post admissions in 2013 , and had 94
cases with neurological sequelae, notably recurrent seizures being the most
common complication[57].
· Touré et al recorded a total number of 31 cases
out of 833 CSF specimens analysed and had Streptococcus pneumoniae,
followed by Neisseria meningitidis being the most common pathogens
[8].
IN CAMEROON
· Sile et al in a study done in Garoua Provincial
Hospital in the North region of Cameroon in 1999, reported bacterial meningitis
to be responsible for 5 % of consultations and 9 % of hospitalisation. Children
less than 5 years affected at 41 % [67].
· Fonkoua et al conducted a study in 2001 at Centre
Louis Pasteur in Yaoundé, demonstrated that the main etiological agents
detected in samples of cerebrospinal fluid sent to this laboratory, were
Streptococcus pneumoniae at 56%, followed by Haemophilus
influenzae 18%, also noting that, the 4 strains of Neisseria
meningitidis of serotypes W135 were found isolated[68]
· Gervaix et al reported in 2012 in a study that only 62
% of theStreptococcuspneumoniaetype in Cameroon are covered by
vaccins, bringing out the question on the certainty of the vaccination against
bacterial meningitis and its impact in this country[69]
· Nguefack et al demonstrated in 2014 in a retrospective
study conducted at YGOPH that the incidence of bacterial meningitis was high in
Cameroon with Haemophilus influenzae being the most common pathogen
responsible of bacterial meningitis in children with 39.2%, followed by
Streptococcus pneumoniae with 31.6% and
28
Neisseria meningitidis least with 10.5%.There was a
high mortality observed with poor prognostic factors as ; age, attitude in
treatment , pathogen incriminated (for this particular study pneumococcal
meningitis) and emphasis was done on the strengthening of routine immunization
on vaccines preventable diseases of infants and children[3]
29
CHAPTER THREE
MATERIALS AND METHODS
III.1) STUDY DESIGN
This was a retrospective- descriptive cross sectional study.
III.2) DURATION OF THE STUDY
The study went on from the 1st December 2018 till
the 31st May 2019, a period of 6 months.
III.3) STUDY SETTING
This study was carried out at the Yaoundé
Gyneco-Obstetric and Pediatric hospital (YGOPH) at the pediatric unit. This
unit is composed of 3 sub units which are; Hospitalisation 1 and 2 of infants
and young children; External consultation with vaccination; and
Neonatalogy.Hospitalisation 1 has 6 rooms and hospitalisation 2 has 7 rooms
with one isolation room for contagious diseases. The unit is also made up of
instruments such as oxygen extractors (4), aspirators (2) and resuscitators
(2).
The service has an administration made of (1) head of service
and (2) deputy head of services ; medical doctors (12) that is paediatricians
(9) and general practitioners (3); State nurses (20) ; assistant nurses , and
cleaners.
III.4) SAMPLING
There was recruitment of files from a period of 1st
of January 2014 to the 31st of December 2018 of children admitted at
YGOPH who fulfilled the inclusion criteria.
III.5) STUDY POPULATION
Children from 1 month of age to 15 years admitted at the
paediatric unit of YGOPH within the study period.
III.5.1) INCLUSION CRITERIA
- Patients from 1 month of age to 15 years admitted for
bacterial meningitis at YGOPH
- Patients who had bacterial examination of CSF with
identification of the pathogen by culture or soluble antigen
- Patients who had their WBC above 10 cells/mm3.
30
III.5.2) EXCLUSION CRITERIA
- Patients in whom bacterial meningitis has not been confirmed
through examination of csf by culture or soluble antigen
- Any file with incomplete information III.6) SAMPLE
SIZE
N=
d2
The sample size will be calculated using the Cochran's formula:
(??1-??:??)??P(1-P)
Where:
- Z1 - á: 2= is the standard normal
variate at 5% type I error (p<0,005),
- P= expected proportion in the population based on previous
studies or pilot studies, for this study, the proportion from NGUEFACK of
1.54%, 2014 was used [3].
-d= Absolute error or precision.
For our study the numerical application of the above formula was
used:
Z1 - á: 2=1.96; P=0.0514%; d=5%. Then
N=1.96*1.96*0.0154(1-
0.0154)/0.05*0.05=23.29
After plugging in all the information in the
equation, we get a minimum of 23files to collect and use.
III.7) MATERIALS
? Materials of data collection and analysis
o Registered files from the paediatric unit
o A laptop
o Microsoft® Office Excel 2016 and CS PRO 7.2
software for data analysis and Microsoft® Office Word 2016 to
keyboard the thesis.
o A 8 GB flash disk
o A 4 GB flash disk
o
31
Office equipment
o Questionnaire for data collection
o Ballpoint ink-pens, pencils, correction fluid and eraser. ?
Human resources:
o The investigator
o Supervisor and co supervisors
o Collaborators (service of archives, paramedical and medical
personnel)
III.8) METHODS
III.8.1) ADMINISTRATIVE AUTHORIZATIONS AND ETHICAL
CLEARANCE
Ethical clearance was obtained from the Ethical Committee of
the Faculty of Health Sciences of the University of Bamenda. Authorization to
carry-out the study at the YGOPH was obtained from the Director General of
YGOPH.
The recruited information's from the questionnaires was held
secret by the investigator in order to maintain data confidentiality taken
throughout the study, and was definitely destroyed through burning after the
data was analyzed and results confirmed.
III.8.2) PATIENT RECRUITMENT
After the approval of the research protocol and the research
authorizations from the different authorities, the personnel in charge of
archives in the pediatric unit were approached. An exhaustive census of all the
children admitted at the pediatric unit with bacterial meningitis within the
study period was done with their admission dates. Then, with information
obtained from the files selection was done according to inclusion criteria. The
children not corresponding to the inclusion criteria were excluded from the
study. The next step was that information from the different files or medical
books was filled into the pre-prepared questionnaires and was kept strictly
confidential.
32
The following information was sought:
- Age
- Gender
- Weight of the child
- Origin (Geographical)
- Class level in school
- Rank among the siblings
- Past history (Immunization calendar, p24 status, underlying
disease, any
contact with someone with meningitis)
- Information on the mother /guardian (age, profession, level of
education,
matrimonial status)
- Manifestation of the disease in the child (convulsions, fever,
kerning,
Brudzinski's, nuchal rigidity, lethargy) and physical
examination
- Laboratory findings on CSF (Isolation of bacterium either by
culture ,
soluble antigens and /or Gram stain)
- Treatment (antibiotherapy proposed)
- Evolution throughout the admission (cured, death)
- Any preventive measure proposed (either on the education on
vaccination
programme which is to be respected as directed or education on
chemoprophylaxis with the rest of the individuals at high risk in the
entourage).
The studied variables included:
? Dependent variables: childhood bacterial meningitis
? Independent variables:
- socio- demographic,
- intervening factors such as immune status
33
III.8.3) DATA MANAGEMENT
The data were collected and recorded on the pre-prepared
questionnaire, where the data in a form designed and coded for study. Pass word
were employed on the PC in order to ensure security and confidentiality of
data.The data were analysed using Microsoft Excel 2016 and CS PRO 7.2 software.
The data were analysed following the analyses plan set by the investigator; and
the results were recorded then discussed accordingly. Evolution was grouped
into three categories: Cured and death.
III.8.3.1) ETHICAL CONSIDERATIONS
The data collected during the study process was kept and
treated confidential, and the prepared questionnaires used to collect the data
were destroyed after the analyses finished. The study was not used for any
personal profit, but for benefits to the general population and also to health
care givers which will use the information for the amelioration of care in our
various health centers and hospitals.
III.9 MANAGERIAL ASPECTS III.9.1) HUMAN RESOURCES
o Supervisor: 01
o Co-supervisors: 02
o Principal Investigator: MAURANE EMMA NDJOCK MBEA
o Collaborators (medical and paramedical personnel)
o A statistician
34
CHAPITRE FOUR
RESULTS
IV.1) INCIDENCE
A total number of 14868 were admitted in the general pediatric
unit of the YGOPH within the studied period, among which 43 cases of bacterial
meningitis were admitted with biological evidence of either leucocytes count
>10 cells /mm3 or positive bacteria culture or soluble antigen, (from which
bacteria were isolated in 16 cases) giving an incidence of 0.3%.
IV.2) SOCIODEMOGRAPHIC CHARACTERISTICS OF PATIENTS
Age groups
14(32.6%)
16

14
12
10
4
8
6
0
2
13(30.2%)
8(18.6%)
2(4.7%)
6(14.0%)
[1-3mths[ [3mths-1yr [ [1yr-3yrs [ [3yrs-5yrs] >5yrs
Figure 6 : Distribution of patients according to age
groups
The age range of 3months - 1year had the highest percentage of
32.6 %, out of the total number of patients admitted for bacterial meningitis.
The mean age of patients admitted was 22.4 months (ranging from 1 - 60
months).
35
Table I: Distribution of patients according to
gender
Sex Number Percentage(%)
Male 19 44.2
Female 24 55.8
Total 43 100
The female sex is noted to have the highest admission with 24
patients among the 43 cases with a sex ratio of 0.8.
IV.2.3) Type of admissions
Type of admission
26(60.5%)
30
25
20
15
10
5
0
17(39.5%)
YGOPH Referral
Figure 7: Distribution of patients according to the type
of admission.
Most of the patients (60.5 %) consulted directly and 39. 5 %
were referred.
36
V.2.4) Distribution of patients according to year of
admission

0.5 0.45 0.4 0.35 0.3 incidence per year 0.25 0.2 0.15 0.1 0.05
0 Year
0.46
0.37
0.3
0.26
|
0.07
|
|
2014
|
2015
|
2016
|
2017
|
2018
|
|
0.37
|
0.46
|
0.07
|
0.3
|
0.26
|
Figure 8: Flow chart illustrating the incidence per year
at YGOPH.
From the year 2014 there was an increase in incidence of 0.37
%, with a total number of 8 cases confirmed with meningitis, to 2015 with an
incidence of 0.46 % with a total number of 12 confirmed cases. Then came a
sudden drop of incidence in 2016 with 0.07% with a number of 2 cases, then
increased as from 2017 with 0.3% with a number of 11 cases and slightly dropped
in 2018 with 0.26 % with 10 confirmed cases.
37
IV.3) CLINICAL PRESENTATION OF BACTERIAL MENINGITIS Table
2: Distribution of clinical presentation according to symptoms.
Symptoms Number Percentage(%)

Neurologic
- Convulsion 26 60.5
- Cevical pain 2 4.7
Digestive
Respiratory
Behavioural
Thermal
Total 41 95.3
otl
Total 23 53.6
Total 8 18.6
Total 31 72.2
Total 10 23.3
- Loss of
consciousness
- Headache
- Diarrhea 10 23.3
- Feeding problems - Vomiting
- Irritability 10 23.3
- Respiratory distress 8 18.6
- Fever 41 95.3
2
7
6
1
4.7
2.3
16.3
14.0
Most patients presented with fever (95.3) % and convulsion
(60.5) % at admission.
38
Table 3: Distribution of neurological clinical
presentation according to signs
Clinical signs Number Percentage(%)
Neck stiffness 9 20.9
Meningeal signs 7 16.3
Bulging fontanella 4 9.3
*Among patients with meningeal signs 7 had both
Kernig and Brudzinski sign.
The clinical sign most found was neck stiffness with 20.9 % of
the total patients admitted, followed by meningeal signs at 16.3 % where both
Kernig and Brudzinski's signs were present in all patients who presented with
meningeal signs that is 100 %.
39
IV.4) PARACLINICAL INVESTIGATION
|
Table 4: Biochemical and cytological aspect of csf
analysis of patients at admission
|
|
Mean#177;SD
|
Min - max
|
|
WBC/??????
|
1181.2#177;6379.0
|
11 - 42000
|
|
Protein g/dL
|
1.30#177;1.15
|
0.1 - 5.1
|
|
Glucose g/L
|
0.49#177;0.21
|
0.16 - 1.10
|
|
RBC
|
1665.8#177;6646.5
|
1 - 36000
|
The means of the WBC were 1181.2#177;6379.0 cells/mm3 with the
minimum being 11 cells /mm3and the maximum 42000 cells /mm3. Proteins had a
mean of 1.30 #177; 1.15g/L with the minimum at 0.1 and maximum at 5.1
g/L.Glucose was 0.49#177;0.21 g/L with the minimum at 0.16 to 1.10 g/L.RBC had
a mean 1665.8#177; 6646.5 cells/mm3 with the minimum at 1 and maximum at 36000
cells/mm3.
40
Table 5: Biochemical and cytologic aspect of csf
analysis of patients at admission
|
Variable
|
Number
|
Percentage (%)
|
|
White blood cells /mm3
|
|
|
|
- [100-1000[
|
22
|
51.2
|
|
- [10-100[
|
19
|
44.2
|
|
- [1000-10000[
|
1
|
2.3
|
|
- =10000
|
1
|
2.3
|
|
-
|
|
|
|
Proteins (g/L) N=22
|
|
|
|
- <0.40
|
6
|
14.0
|
|
- 0.40 - 0.79
|
2
|
4.7
|
|
- 0.80 - 0.99
|
1
|
2.3
|
|
- =1
|
13
|
30.2
|
|
Glucose (g /L) N=22
|
|
|
|
- <0.4
|
9
|
20.9
|
|
- 0.4-0.6
|
8
|
18.6
|
|
5
|
11.6
|
|
- >0.6
|
|
|
*N=number of patients who had CSF analysis done
for both biochemistry and cytology.
The biochemical and cytologic aspects of the CSF analysis showed
in this study that most of the patients that were confirmed with meningitis had
the WBC from 101000 cells /mm3 at 95.4 %.
Then 22 patients had their CSF biochemistry done and, among them
13 had proteins =1 g/L at 30.2 % and 9 patients had glucose <0.4 g/L with a
high percentage of 20.9 %.
41
IV.5) ETIOLOGIES OF BACTERIAL MENINGITIS

N. meningitidis
4 (25%)
Group B Strep
1 (6%)
Salmonella
1 (6%)
S. pneumoniae
10 (63%)
*Culture = 6 patients
* Soluble antigen = 7 patients * Gram stain = 10
patients
*It is seen that the means of identification of
bacterium indicates more than 16 patients but it is due to the fact that a
bacterium was identified by both Gram stain and soluble antigen.
Streptococcus pneumoniae was the most predominant
bacterium isolated with 56 %, followed by Neisseria meningitidis at 33
%. Other bacteria found were Salmonella and Group-B streptococcus
each at a lower percentage of 6 %.
42
Table 6: Distribution of pathogens according to
age
|
GERM
|
[1 - 3[ month s
N (%)
|
[3 - 12[ months N (%)
|
[12 -36[ months N (%)
|
[36-60[
months
N (%)
|
=60 months N(%)
|
Total
|
|
2
|
5
|
2
|
0
|
1
|
10
|
|
S.pneumoniae
|
(15.4)
|
(35.7)
|
(25.0)
|
(0.0)
|
(16.7)
|
(23.3)
|
|
0
|
1
|
0
|
0
|
0
|
1
|
|
(0.0)
|
(7.1)
|
(0.0)
|
(0.0)
|
(0.0)
|
(2.3)
|
|
GBS
|
|
|
|
|
|
|
|
N.meningitidi
|
1
|
1
|
0
|
1
|
1
|
4
|
|
s
|
(7.7)
|
7(.1)
|
(0.0)
|
(50.0)
|
(16.7)
|
(9.3)
|
|
Salmonella
|
0
|
1
|
0
|
0
|
0
|
1
|
|
(0.0)
|
(7.1)
|
(0.0)
|
(0.0)
|
(0.0)
|
(2.3)
|
|
No culture nor soluble
antigen done
|
1
(7.7)
|
2
(14.3)
|
3
(37.5)
|
0
(0.0)
|
1
(16.7)
|
7
(16.3)
|
|
Sterile
|
9
|
4
|
3
|
1
|
3
|
20
|
|
(69.2)
|
(28.6)
|
(37.5)
|
(50.0)
|
(50.0)
|
(46.5)
|
|
13
|
14
|
8
|
2
|
6
|
43
|
|
TOTAL
|
(100.
|
(100.0
|
(100.0
|
(100.0
|
(100.0
|
(100.0
|
|
0)
|
)
|
)
|
)
|
)
|
)
|
It is seen that among the 43 cases of bacterial meningitis,
bacteria were isolated in 16 cases with a total percentage of 37.2% of patients
who did either the culture or soluble antigen for the diagnosis of bacterial
meningitis. From the 16 cases, we had Strep pneumoniae which was
higher present at 23.3 % at age range 3months to 12 months more affected.
Neisseriameningitidis follows with 9.3% and evenly distributed amongst the
age group. It should also be noted that 46.5 % of the cases had sterile CSF.
43
IV.6) HOSPITAL OUTCOME
Table 7: Distribution of complications found during
admission
|
Complication
|
|
Number
|
Percentage(%)
|
|
Respiratorydistress
|
|
9
|
20.9
|
|
Anemia
|
|
7
|
16.3
|
|
Brain abcess
|
|
6
|
14.0
|
|
Status epilepticus
|
|
5
|
11.6
|
|
Dehydration
|
|
5
|
11.6
|
|
Motor deficit
|
|
4
|
9.3
|
|
Intracranial hypertension
|
|
4
|
9.3
|
|
Hydrocephalus
Others (strabismus,
retardation)
|
psychomotor
|
4
2
|
9.3
4.7
|
|
Cerebral empyema
|
|
2
|
4.7
|
Most of the patients with bacterial meningitis developed
respiratory distress in the course of admission with a percentage of 20.9 %,
followed by anemia with a percentage of 16.3 %. Brainabscess also being an
important complication in meningitis was present at 14.0%.
44
Table 8: Distribution of patients according to outcome
during admission
Outcome Number Percentage(%)
Cured 41 97.6
Death 1 2.4
Total 42 100
Most patients admitted for meningitis 41 were cured at 95.3 %
and among those, 25 had complications at 61.0 %.
We recorded 1 death, and 1 patient went against medical
advice.
45
Table 9: Distribution of patients according to sequelae
at time of discharge
Sequelae Number Percentage(%)
Hydrocephalus 4 9.3
Tetraparesis 2 4.3
Hemiparesis 1 2.3
Facial paralysis 1 2.3
Psychomotorregression 1 2.3
Hydrocephalus was the most frequent sequelae at time of
discharged at 9.3 % of all the complications found.
46
Table 10: Distribution of patients according to
treatment recieved for sequalae
|
Sequelae
|
N(%)
|
Treatment
|
N
|
|
Hydrocephalus
|
4(9.3)
|
Neurosurgery
|
2
|
|
Tetraparesis
|
2(4.3)
|
Physiotherapy
|
1
|
|
Hemiparesis
|
1(2.3)
|
-
|
|
|
Facial paralysis
|
1(2.3)
|
-
|
|
|
Psychomotorregression
|
1(2.3)
|
Physiotherapy
|
1
|
Among the patients withsequelae, 2 undergone surgery and 2
undergone physiotherapy.
47
CHAPITRE FIVE
DISCUSSION
Our main objective was to identify the common pathogens that
cause bacterial meningitis in children at the Pediatric unit in YGOPH and to
describe their hospital outcome. At the end of our study, we were able to
identify these pathogens causing bacterial meningitis in children and described
the hospital outcome.
V.1) INCIDENCE
A total number of 43 patients were admitted at the general
pediatric unit for bacterial meningitis with biological confirmation from the
1st of January 2014 till the 31st of December 2018 giving an incidence of 0.3
%. This incidence is similar to that of Shingoh et al in Japan that had an
incidence of 0.37 %[63] .Our incidence for bacterial
meningitis is far lower than what Nguefack et al had in 2014 in YGOPH where
bacterial meningitis was 1.54 % of the total admissions in the general
pediatric unit[3].This large difference could be explained
first by the problem of lost files from the archives .Secondly , the difference
could be explained by the vaccination programme in Cameroon which was
introduced since 1976, became operational in all the regions in 1982 and covers
children from 0-11 months of age against infectious diseases[73]
. In that same line, in a study done at YGOPH in 2016, there was
vaccination completeness in children aged from 0-11 months at 96.3 %
[73]. Koko et al in Libreville, Gabon also had a higher
incidence of 1.2 % [65], thus our incidence tends to be much
lower than most incidences of bacterial meningitis in Africa and even for those
in developed countries; 0.70% in Finland in 2014[62].
V.2) SOCIODEMOGRAPHIC CHARACTERISTICS
The average age in our study was 22.4 months, with most
patients admitted for bacterial meningitis being less than 12 months of age.
These are similar to Nguefack et al who had more patients with 2 months - 1
year of age [3]. Similar results were observed with Mohammed
in Al-Ramadi with more patients being less than 1 year of age, but our results
are contradictory to those of Campagne who had instead less patients of
bacterial meningitis with less than 1 year of age [33][
64].Fayyaz also had less patients of less than 1 year of age
[70].
48
The sex ratio was 0.79 in our study. There was a predominance
of female sex admissions of 56 %.This result is similar to that of Almuneef et
al in Saudi Arabia which had 36 females over 34
males[59].Contradictory result is gotten from Sile et al who
had a sex ratio of 1.65 with a predominance in male sex in Garoua Provincial
hospital[67] , also with Franck - Briggs who had male
predominance of 58 in Nigeria[57].Otero et al had in Columbia
a predominance in the male sex of 61.4 %[72] .Thus any
predominance of either sex varies with each study.
There was more direct entry in YGOPH than referral in our
study with 60.5 %. This result can be explained by the fact that YGOPH is a
tertiary and a reference hospital which has the capacity to take care of
emergencies.Therefore, patients will prefer to start consultations directly in
such settings especially as they consider some symptoms as a sign of
fatality.
V.3) CLINICAL PRESENTATION OF PATIENTS
The presentations in our study were divided into two; symptoms
and signs. The most predominant symptom presented in children with bacterial
meningitis was fever at 95.3%, and is similar to that of Nguefack et al who had
fever as the main symptom at 98.8 % at YGOPH [3]. This result
is similar to that of Almuneef et al who had fever as the main symptom at 86 %
[59]. This result is also the same as the one from Heydari who
had fever as the most common symptom at 94.44 % [48]. The high
appearance of fever may be explained by the fact that most infectious diseases
start manifesting with high temperature before any other symptom, and the
knowledge on the fatality of fever on children prompts consultation with this
earliest sign. Zewdie in Ethiopia instead had feeding intolerance at 76.6 % as
the main symptom in neonates [5].Diarrhea was present in our
study at 23.3 % as a digestive manifestation which is rare in meningitis, but
in this setting the bacterium causing gastro enteritis is very common and could
easily find its way into the central nervous system through hematogenous
route.
The most present sign was neck stiffness with 20.9 % which is
contrary to that of Heydari who had fever as the main sign at 94.44 %
[48]. The result is also contradictory to that of Johnson who
had drowsiness/coma at 50.0 % as the predominant clinical sign
[10].
49
V.4) PARACLINICAL INVESTIGATIONS
The biochemical and hematologic analyses of cerebrospinal
fluid in our study are suggestive of bacterial meningitis, with the WBC mean of
1181.2 which is similar to the results gotten from Heydari who had high WBC
count [48]. This mean result of WBC could be explained by the
fact that most of the patients received antibiotic treatments before the lumbar
puncture was performed.
The proteins found in our study were high that is,=1 g/L
predominantly at 30.2 % which is similar to Heydari that had >0.4g/L at
47.2% [48], confirming the presence of bacterial meningitis
,where it is known that proteins tend to increase.
The glucose of <0.4g/L was most present at 20.9 % in our
study and is similar to that obtained with Heydari who had the same quantity at
a higher percentage of 75%, proving the diagnosis of bacterial meningitis
[48].
From our study Streptococcus pneumoniae was the
pathogen mostly found at 63 %, followed by Neisseria meningitidis at
25.0%. These results are contradictory with that of Nguefack et al at YGOPH in
2014 that had Haemophilus influenzae as the predominant pathogen at
39.2 %, followed by Streptococcus pneumoniae at 31.6 % [3].
Nevertheless, our results are similar to that of Fonkoua et al who had
Streptococcus pneumoniae at centre Pasteur in Yaoundé at 56 %
[68]. There is also a similarity with that of Mullan et al in
Botswana that found Streptococcus pneumoniae predominantly with n
=125[66].Touré et al in Bouaké had the same
results as ours with Streptococcus pneumoniae at 48.4 % as predominant
followed by Neisseria meningitidis 16.1 %[8].Otero in
Columbia also had Streptococcus pneumoniae as the predominant pathogen
with a percentage of 11.4 %[72].These discrepancies in results
are justified by the fact that each setting in which the study was done had a
well-planned vaccination programme , which is probably implemented correctly
especially for the Hib vaccine which is proven to be efficient.
The age group of 3 months to 12 months has the highest
percentage of bacterial meningitis caused by Streptococcus pneumoniae
at 35.7 %. This is different to that of Nguefack et al at YGOPH who had
most patients from 2 months - 1 year affected with Haemophilus influenzae
n= 43[3].Touré et al had S. pneumonia
predominance in a higher age group that is 13 - 60 months in
Bouaké[8].These results could be explained
50
by the fact that vaccination completeness in YGOPH was high
for the vaccines at 97.1%[73]explaining the absence of
Haemophilus influenzae in our study , with the introduction of the
Haemophilus vaccine into the PEV in 2009 and the pneumococcal vaccin in 2010 ,
in Cameroon[73].
V.5) HOSPITAL OUTCOME OF BACTERIAL MENINGITIS IN
CHILDREN
We recorded in our study 1 death representing 2.4 % of all the
children admitted for bacterial meningitis. This is very low compared to that
of Nguefack et al at YGOPH in 2014 with 17 deaths that is 53.1 % [3].
Koko in Gabon had much higher with 62 % [65].
However, our result had similarities with that of Wee LY et al in
Singapore who recorded 6 % of death cases [71]. Chandran had a
much lower percentage of 0.4% [6]. This low record of ours is
probable due to the early death of patients that were suspected of having
meningitis at entry, who died before any confirmatory examination notably
lumbar puncture could be performed probably because they presented with
contraindications.
We found that respiratory distress was the most common
complication of bacterial meningitis during hospitalization at 20.9 % at the
general pediatric unit at the YGOPH, followed by anemia with 16.3 %. We also
noted dehydration and status epilepticus as complications of meningitis both at
11.6 %. It is different from what Franco-Paredes and Nguefack had both with
seizure disorders and status epileticus as their main complication of bacterial
meningitis at 37 % and 54.7 % respectively[3][60].This result
can be explained by the fact that Streptococcus pneumoniae was the
predominant pathogen found , thus it is obvious for these patients to have
respiratory problems. The anemia can be justified by the fact that there must
have been an associated disease like malaria which is known to cause anemia
.Dehydration being present as a complication can be explained by the symptom of
fever, where fluid tends to be lost through sweat.
Hydrocephalus was the most common sequelae in the study at 9.3
% which is contradictory to that of Nguefack et al who had psychomotor
regression as the highest sequelae at 2.9 %3]. Sile had at the
Garoua provincial hospital 1 case of paraplegia [67].
Among the 4 patients that had hydrocephalus from our study, 2
had neurosurgery as treatment. This is probably due to poor financial
conditions that disabled the 2 others from benefiting from the treatment.Among
patients who had psychomotor regression (1)
51
and tetra paresis (2), 2 had physiotherapy done for their
rehabilitation, explained by probable poor financial conditions for the one
that could not afford for the service.
? LIMITATIONS OF THE STUDY
We encountered various difficulties of which some are
naturally found in every retrospective study that is: files with incomplete
information, files that were not exploitable, and many files were lost from the
archives because most of these files didn't return when they left this service
for another unit in the hospital. There was a problem of uniformity among the
health personnel too in taking clinical observations notably for the
diagnosis.
Also considering the fact that there were a limited number of
pathogens available, the antibiogramme sensitivity analysis could not be
done.
CONCLUSION
At the end of this study, the different specific objectives
have been attained. We can therefore come to the conclusion that:
> The incidence of bacterial meningitis in children at the
YGOPH is 0.3%.
> The most common etiologies responsible for bacterial
meningitis in YGOPH are Streptococcus pneumoniae at 63% and
Neisseria meningitidis at 25 % and presented more in children <12
months of age .
> Most children presented at the consultation with fever as
the predominant symptom at 95.3%, followed by convulsions at 60.5 %.On clinical
examination, neck stiffness was the most common sign at 20.9%, followed by
meningeal signs at 16.3%.
> The mortality was 2.4 %, and 97.6 % of patients left the
hospital alive .Among the 97.6 % patients that left the hospital alive, 61.5%
had neurological complications.
RECOMMENDATIONS
Our conclusions above enable us to do the following
recommendations;
? TO THE MINISTRY OF PUBLIC HEALTH
- Re-enforce vaccination campaigns in children all over the
country.
52
? YAOUNDE GYNECO-OBSTETRIC AND PEDIATRIC
HOSPITAL
- Re-enforce information towards infectious diseases especially
on meningitis.
- Re-enforce information, education and communication on
vaccinations of children.
53
REFERENCES
1. Oordt-Speets AM, Bolijn R, van Hoorn RC, Bhavsar
A, Kyaw MH.
Global etiology of bacterial meningitis: A systematic
review and meta-analysis. PloS One. 2018 Jun
11;13(6):e0198772.
2. Habtamu A, Sadikalmahdi H, Chelkeba L.
Childhood bacterial
meninigitis: Antimicrobial use pattern and
treatment outcomes: a prospective observational study.ClinPract. 2018;
15(SI):587-02.
3. Nguefack S, Chiabi A, Enoh J, Mah E, Kamga KK,
Tatah S,et al.
Etiologies and Outcome of Children with Purulent
Meningitis at the Yaounde Gyneco-Obstetric and Pediatric Hospital (Cameroon).
Open J Pediatr. 2014 Nov 3; 4(04):269-75.
4. Curtis S, Stobart K, Vandermeer B, Simel DL,
Klassen T. Clinical
features suggestive of meningitis in children:
a systematic review of prospective data. Pediatr. 2010 Nov 1; 126(5):952-60.
5. Zewdie AT. Prevalence,aetiology and
antimicrobial susceptibility of bacterial
neonatal meningitis at Tikur
Ambessa specialized Hospital, Addis Abbeba , Ethiopia
[Thesis].[Nairobi]:University of Nairobi; 2011.
6. Chandran A, Herbert H, Misurski D, Santosham M.
Long-term sequelae
of childhood bacterial meningitis: an
underappreciated problem.Pediatr Infect Dis J. 2011 Jan 1; 30(1):3-6.
7. Pelkonen T, Roine I, Monteiro L, Correia M,
Pitkäranta A, Bernardino
L et al. Risk factors for death and
severe neurological sequelae in childhood bacterial meningitis in sub-Saharan
Africa. Clin Infect Dis. 2009 Apr 15; 48(8):1107-10.
8. Touré FS, Kouame S, Tia H, Monemo P,
Cissé A, Diané B, et al.
Epidemiology of paediatric
meningitis in central Côte d'Ivoire after the implementation of
Haemophilus influenzae type b vaccination. Newmicrobiol. 2017 Jul 1;
40(3):170-4.
9. Robbins JB, Schneerson R, Gotschlich EC, Mohammed
I, Nasidi A,
Chippaux JP,et al. Meningococcal meningitis in
sub-Saharan Africa: the case for mass and routine vaccination with available
polysaccharide vaccines. Bull World Health Organ. 2003; 81:745-50.
10.
54
Johnson AW, Adedoyin OT, Abdul-Karim AA, Olanrewaju
AW. Childhood pyogenic meningitis: clinical and investigative
indicators of etiology and outcome. J Natl Med Assoc. 2007 Aug; 99(8):937.
11. Martin Health. United Nations Sustainable
Development; November 2018. Available from
https://www.un.org/sustainabledevelopment/health
[Assessed 06 Dec 2018]
12. WHO. Weekly epidemiological Record Who.
2011.Available from
https://www.who.int/wer/2011/wer8647
[Assessed 12 Dec 2018]
13. WHO. Epidemic meningitis control in
countries of the African meningitis belt Who.2018.Availablefrom
https://apps.who.int/iris/handle/10665/272297.
[Assessed Dec 2018]
14. Nadel S. Prospects for eradication of
meningococcal disease. Arch Dis child. 2012 Nov 1; 97(11):993-8.
15. WHO (CDS/CSR/EDC/99, 7.Laboratory methods
for the diagnosis of meningitis caused by Neisseria meningitidis ,
Streptococcus pneumoniae and Haemophilus influenza
who.1999.AvailablefromWhqlibdoc.who.int/hq/2011/WHO_IVB_11.09[Asse ssed 12 Dec
2018].
16. CDC.Pediatric. Bacterial meningitis
Surveillance-AfricanRegion(2002-2008).Available from
https://
wwwnc.cdc.gov>12-0375_article [Assessed Feb 2019]
17. Chacon-Cruz E, Alvelais-Palacios JA,
Lopatynsky-Reyes EZ, Rodriguez-Valencia JA, and Volker-Soberanes ML.
Meningococcal Disease in Children: Eleven Years of Active Surveillance in a
Mexican Hospital and the Need for Vaccination in the Tijuana Region. J Infec
Dis Treat. 2017;3:1.
18. Peltola H. Worldwide Haemophilus
influenzae type b disease at the beginning of the 21st century: global analysis
of the disease burden 25 years after the use of the polysaccharide vaccine and
a decade after the advent of conjugates. Clin microbiol Rev. 2000 Apr
1;13(2):302-17.
19. Gurley ES, Hossain MJ, Montgomery SP, Petersen
LR, Sejvar JJ, Mayer LW,et al. Etiologies of bacterial meningitis in
Bangladesh: results from a hospital-based study. Am J Trop Med.Hyg. 2009 Sep 1;
81(3):475-83.
20.
55
Immunopedia .Org. Streptococcal pneumoniae
meningitis 2011 -2019.
Availablefrom
https://www.immunopaedia.org.za/immunology/archive/immu
ne-evasion/blood-brain-barrier/iga-complement/streptococcal-pneumoniae-meningitis[Assessed
02 Feb 2019].
21. Mellroth P , Daniels R, Eberhardt A, Ronnlund D ,
Blom H, Widengren J et al. Lyt A, major autolysin of Streptococcus
pneumoniae , requires access to nascent peptidoglycan.J Biol Chem .2012 Mar
30;287(14):11018-29.
22. Tacon CL, Flower O. Diagnosis and
management of bacterial meningitis in
the paediatric population: a review.
Emerg Med Int. 2012;2012 :320-09.
23. Chávez-Bueno S, McCracken GH.
Bacterial meningitis in children. Pediatr
Clin. 2005 Jun 1;
52(3):795-10.
24. Jauneikaite E, Mary Carnon Jefferies J, William
VereChurton N, Tzer Pin Lin R, Lloyd Hibberd M, Charles Clarke S.
Genetic diversity of Streptococcus pneumoniae causing meningitis and
sepsis in Singapore during the first year of PCV7 implementation. Emerg
Microbes Infect. 2014 Jan 1;3(1):1-7.
25. Aviq. Infection invasive à
Haemophilus influenza type b. Juillet 2016.
Available from
https://www.wiv-isp.be>H_influenzae
[assessed May 2019]
26. Rosadini C. Roles of secreted virulence
factors in pathogenecity of
Haemophilus influenzae: A Dissertation, United
States of America [Thesis].[Massachusetts]:University of Massachusetts medical
school;2011.
27. Naik DG, Seyoum M. Haemophilus
influenzae type b meningitis in children,
Eritrea. Emerg Infect Dis. 2004
Jan;10(1):155-6.
28. Greenberg-Kushnir N, Haskin O, Yarden-Bilavsky H,
Amir J, Bilavsky E. Haemophilusinfluenzae type b meningitis in the
short period after vaccination: a reminder of the phenomenon of apparent
vaccine failure. Case Rep Infect Dis. 2012;2012 : 950-07.
29. Manchanda V, Gupta S, Bhalla P.
Meningococcal disease: history,
epidemiology, pathogenesis,
clinical manifestations, diagnosis, antimicrobial susceptibility and
prevention. Indian J Med Microbiol. 2006 Jan 1;24(1):7-19.
30. Hart CA, Thomson AP. Meningococcal
disease and its management in children. BMJ. 2006 Sep 28; 333(7570):685-90.
31.
56
Thigpen MC, Whitney CG, Messonnier NE, Zell ER,
Lynfield R, HadlerJL,et al. Bacterial meningitis in the United States,
1998-2007.Engl J Med. 2011 May 26; 364(21):2016-25.
32. WHO. Meningococcal vaccines, November2011,
Weekly Epidemiological
Record[Internet]Who.2011.Available from
https://www.who.int/wer/2011/wer8647
[Assessed 03 Feb 2019].
33. Rouphael N.G, Stephens D.S. Neisseria
meningitidis: Biology, microbiology
and epidemiology. Methods Mol Biol.2012;
799: 1-20.
34. Al-Ani MM. Risk Factors of Meningitis in
Children Under Five Years in Al-
Ramadi Maternity and Children Hospital.
Al-Anbar Med J. 2009;7(1):76-84.
35. Al Jarousha AM, Al Afifi A. Epidemiology
and risk factors associated with
developing bacterial meningitis among
children in Gaza Strip. Iran J PublHealth. 2014 Sep;43(9):1176-83.
36. Shapiro ED, Aaron NH, Wald ER, Chiponis D.
Risk factors for development of bacterial meningitis among children
with occult bacteremia. J Pediatr. 1986 Jul 1;109(1):15-9.
37. Borrow R, Caugant DA, Ceyhan M, Christensen H,
Dinleyici EC, Findlow J, et al. Meningococcal disease in the Middle
East and Africa: Findings and updates from the Global Meningococcal Initiative.
J Infect. 2017 Jul 1;75(1):1-1.
38. Hoffman O, Weber JR. Pathophysiology and
treatment of bacterial
meningitis. Ther Adv Neurol Discord. 2009
Nov;2(6):401-12.
39. Cohen ML. Changing patterns of infectious disease.
Nature.2000 Aug;406(6797):762-7.
40. Leib SL, Täuber MG. Pathogenesis of
bacterial meningitis. Infect Dis Clin
North Am. 1999 Sep 1; 13(3):527-48.
41. Baines P, Reilly P, Gill. Paediatric
meningitis: Clinical features and
diagnosis.ClinPharm .2009 Aug; 307-10.
42. Schulga P, Grattan R, Napier C, Babiker MO.
How to use... lumbar puncture in children. Arch Dis Child EducPract.
2015 Oct 1;100(5):264-71.
43. Hasbun R. Update and advances in
community acquired bacterial
meningitis.Curr Opin Infect Dis. 2019 Jun 1;
32(3):233-8.
44.
57
Gorn M, Kunkov S, Crain EF. Prospective
investigation of a novel
ultrasound-assisted lumbar puncture technique on
infants in the pediatric emergency department. Acad Emerg Med. 2017
Jan;24(1):6-12.
45. Saberi A., Roudbary S.A., Ghayeghran A., Kazemi
S.,Hosseininezhad
M.Diagnosis of meningitis caused by pathogenic
microorganism using magnetic resonance imaging ; A systematic review.Basic clin
Neurosci.2018 Apr ;9(2):736-86
46. Velissaris D, Pintea M, Pantzaris N, Spatha E,
Karamouzos V,
PierrakosC,et al. The role of procalcitonin in the
diagnosis of meningitis: a literature review. J Clin Med. 2018 Jun;7(6):148.
47. Swann O, Everett DB, Furyk JS, Harrison EM,
Msukwa MT,Heyderman RS,et al. Bacterial meningitis in Malawian
infants< 2 months of age: etiology and susceptibility to World Health
Organization first-line antibiotics. Pediatr Infect Dis J. 2014 Jun;
33(6):560-65.
48. WHO. Vaccination Who.2018.Available from
https://www.Who.Int/ immunization/p[Assessed on Aug 2019]
49. Makwana N., Riordan F. Bacterial
meningitis: the impact of vaccination
.CNS Drugs.2007.
50. Davis S., Feikin D., Johnson H.The
effect of Hib and pneumococcal
conjugates vaccines on childhood meningitis
mortality: A systematic review. BMC Public Health. 2013.
51. CDC. Pneumococcal vaccins. Available
from
https://www.cdc .gov>vpd
>
pneumo.[Assessed Aug 2019]
52. WHO. Pneumococcal vaccins
Who.2018.Available from
https://www.Who.Int >vaccines
pneumo.[Assessed Aug 2019]
53. WHO.Meningococcal vaccines Who. 2018.
Available from
https://www.Who.Int > Ith >
vaccines >meningitis.[Assessed Aug 2019]
54. WHO.Haemophilus influenza vaccins
Who.2015. Available from https://
www.Who.Int >diseases >hib.[Assessed
Aug 2019]
55. Sáez-Llorens X, McCracken Jr GH.
Bacterial meningitis in children.
Lancet. 2003 Jun
21;361(9375):2139-48.
56.
58
Heydari B, Khalili H, Karimzadeh I, Emadi-Kochak H.
Clinical,
paraclinical, and antimicrobial resistance features of
community-acquired acute bacterial meningitis at a large infectious diseases
ward in Tehran, Iran.Iran J Pharm Res. 2016;15(1):347-54.
57. Control and prevention of meningococcal
Disease.Recommendations of the
Advisory committee on immunization.
1997 Feb.14; 46(RR-5): 1-10.
58. De Souza AL, Sztajnbok J, Seguro AC.
Cerebellar hemorrhage as an atypical complication of meningococcal
meningitis. Int J Infect Dis. 2008 Sep 1;12(5):558-9.
59. Frank-Briggs AI, Alikor EA. Long term
neurological complications of
bacterial meningitis in Nigerian children.
Niger J Paed. 2013;40(3):295-8.
60. Mazankova LN, Milovanova OA, Moiseenkova DA,
Soldatova IA, Mikhalinova EP. Neurological presentations of bacterial
meningitis in children: current possibilities of diagnosis and treatment.
ZhNevrolPsikhiatr Im S SKorsakova. 2016;116(6):4-9.
61. Almuneef M, Memish Z, Khan Y, Kagallwala A,
Alshaalan M. Childhood bacterial meningitis in Saudi Arabia. J Infect.
1998 Mar 1;36(2):157-60.
62. Franco-Paredes C, Lammoglia L, Hernández
I, Santos-Preciado JI.
Epidemiology and outcomes of bacterial
meningitis in Mexican children: 10-year experience (1993-2003). Int J
Infect Dis. 2008 Jul 1;12(4):380-6.
63. Le Saux N. Antimicrobial stewardship in
daily practice: Managing an
important resource. Canad J Infect Dis Med
Microbiol. 2014;25(5):241-5.
64. Polkowska A, Toropainen M, Ollgren J,
Lyytikäinen O, Nuorti JP. Bacterial meningitis in Finland,
1995-2014: a population-based observational study. BMJ Open. 2017 May
1;7(5):e015080.
65. Shinjoh M, Yamaguchi Y, Iwata S.
Pediatric bacterial meningitis in
Japan,
2013-2015-3-5 years after the wide use of
Haemophilusinfluenzae type b and Streptococcus pneumoniae conjugated vaccines.
J Infect Chem. 2017 Jul 1;23(7):427-38.
66. Campagne G, Schuchat A, Djibo S, Ousseini A,
Cisse L, Chippaux JP. Epidemiology of bacterial meningitis in Niamey,
Niger, 1981-96. Bull World Health Organ. 1999;77(6):499-08.
67.
59
Koko J, Batsielili S, Dufillot D, Kani F, Gahouma D,
Moussavou A. Les
méningites bactériennes de l'enfant
à Libreville, Gabon. Aspects épidémiologiques,
thérapeutiques et évolutifs. Med Mal Infect. 2000 Jan
1;30(1):50-6.
68. Mullan PC, Steenhoff AP, Draper H, Wedin T,
Bafana M, AnabwaniG,et al. Etiology of meningitis among patients
admitted to a tertiary referral hospital in Botswana. Pediatr Infect Dis J.
2011 Jul 1;30(7):620-2.
69. Mefo HS, Sile H, Mbonda E, Fezeu R, Fonkoua MC.
Les méningites purulentes de l'enfant au Nord Cameroun: aspects
cliniques, bactériologiques et thérapeutiques. MédAfr
Noire. 1999;46(1):15 -20.
70. Fonkoua MC, Cunin P, Sorlin P, Musi J, Martin PM.
Bacterial meningitis
in Yaounde (Cameroon) in 1999-2000. Bull Soc
PatholExot(1990). 2001 Nov;94(4):300-3.
71. Gervaix A, Taguebue J, Bescher BN, Corbeil J,
Raymond F, AlcobaG,et al. Bacterial meningitis and pneumococcal
serotype distribution in children in Cameroon. Pediatr Infect Dis J. 2012 Oct
1;31(10):1084-7.
72. Fayyaz J, Rehman A, Hamid A, Khursheed M, Zia N,
Feroze A. Age related clinical manifestation of acute bacterial
meningitis in children. J Pak Med Assoc. 2014;64(3):296.
73. Otero Flórez JA, Gómez Navas MD,
Cornejo Ochoa JW, Cabrera Hemer DN. Clinical and paraclinical
characteristics in children with acute bacterial meningitis at the Hospital
Universitario San Vicente Fundación, in Medellín, Colombia.
2011-2015: descriptive-retrospective study. ActaNeurol Colom. 2017
Jun;33(2):84-93.
74. Wee LY, Tanugroho RR, Thoon KC, Chong CY, Choong
CT, Krishnamoorthy S,et al. A 15-year retrospective analysis of
prognostic factors in childhood bacterial meningitis. Acta Paediatr. 2016
Jan;105(1):e22-9.
75. Njapndounke T. Determinants de la
completitude vaccinale chez les enfants
ages de 0 à 11 mois à
L'hopitalGyneco-Obstetric et pédiatrique de Yaoundé, Cameroun
[Thèse].[Yaoundé]: Université de Yde 1;2016.
60
APPENDIX A : AUTORISATION OF THE YGOPH

APPENDIX B : QUESTIONNAIRE
A. IDENTIFICATION OF THE CHILD
Questionnaire N° File N°
Date of admission Weight
Date of birth Age
Sex M F
School level: pre-school primary secondary High school
Origin
Type of admission:CME/FCB Referred
Reason for admission
B. PAST HISTORY OF THE CHILD
1) Immunization calendar up to date yes No
i) If No, which vaccines lacks?
2) P24 statusyes No
i)If yes on treatment
3) Underlying disease yes No
i) If yes what disease
ii) Undergoing treatment for the
disease
4) Any contact with someone with meningitis before admission? Yes
No
C. INFORMATION ON THE PARENT / GUARDIAN
i)MOTHER
Age (years):<20 (20-34) >34
Profession: Liberal Non-liberal Student or pupil Not employed
Level of education: primary secondary higher education
illiterate
Matrimonial status: married single
Past medical history Region of
origin ..
ii)FATHER

Age (years):<20 (20-34) >34
Profession: Liberal Non-liberal Student or pupil Not employed
Level of education: primary secondary higher education
illiterate
Matrimonial status: married single
Past medical history Region of
origin ..
D. MANIFESTATION OF THE DISEASE ON THE CHILD

Convulsion yes No
Fever yes No
Kerning sign yes No
Brudzinski'ssign yes No
Nuchal rigidity yes No
Lethargy yes No
Bulging fontanelle yes No
Feeding problems yes No
Behavioural changes yes No
Respiratory manifestations yes No
i) If Yes which one
Digestive manifestations yes No
Others
E. LABORATORY FINDINGS ON CSF
1) Macroscopic examination of the
CSF/Appearance ..
2) CSF Biochemistry characteristics
Proteins g/dl
Glucose mmol/L
3) Cytology aspect of the CSF
WBC count cells/ml
RBC count cells/ml
4) Gram stain, bacteria isolated yes No
i) If yes what findings
5) Any CSF culture done yes No
If yes which growth was obtained
6)Soluble Antigens yes No
i)If Yes
F. OUTCOME DURING ADMISSION
Any complications yes No
i) If any, which one(s)
ii) Treatment received for the
complications
ii) Mortality yes No
Comorbidities: yes No .If yes which one(s)
G. EVOLUTION
cured Died Discharged against medical advice
H. TREATMENT RECEIVED i) Antibiotherapy
cephalosporins .Others
ii)Adjuvant therapy
Steroids anticonvulsant antipyretics
Iii) Fluid
I. PREVENTIVE MEARSURE
Preventive measures proposed? Yes No
i) If yes, which one?
Education on vaccination chemoprophylaxis of child at risk
| 


