INTRODUCTION
As recommended for our professional master's
degree program, we carried out studies in organizations which are aimed at
solving specific problems. It is for this reason that we carried out our
research in Douala for a period of six months starting from 02nd
July to 31st December 2012 within LES LABORATOIRES BIOPHARMA S.A.
I.Z MAGZI BASSA P.O BOX 1674 DOUALA. Our topic is titled
«EVALUATION OF THE LEVEL OF SAFETY TO IMPROVE ITS THE CULTURE WITHIN
BIOPHARMA». What motivated us to choose the above topic was the fact
that our company is growing very fast but safety standards are not visibly
following the same trend. The hypothesis «encouraging a safety culture by
putting in place a health and safety committee - HSE within Biopharma followed
by specialized trainings, could help us to ameliorate productivity.
What is the current situation of safety
culture; in other words, we verify whether our top management is committed
(inquiry) as well as the personnel (questionnaire), existence of a health and
safety committee and the available means of the firm.
To provide answers, we shall first of all give
some briefs on safety culture; assess the present situation in the company
followed by some examples of companies practicing this culture. Finally we
shall propose a health and safety policy for the company to its General Manager
for approval.
The true `health' of the safety of any
organisation is primarily defined by the frequency of key day-to-day behaviours
(frontline and management) and the extent to which these are encouraged and
supported by an effective and flexible safety management system. The shared
belief in the importance of safety, the extent to which an organisation
actively strives to ensure health and safety is done properly and always given
a high priority, is what defines a positive safety culture. Safety is for
everybody, thus visitors, clients, customers, contractors, friends and family
at any workplace have work health and safety (WHS) responsibilities and must
comply with any reasonable work health and safety instructions at the workplace
and take reasonable care not to put themselves or others at risk directly or
indirectly. There is a need for leaders to know how to manage safety on a day
to day basis throughout their areas of responsibility.
Today, it has generally become accepted that a high proportion
of accidents, incidents and near misses in companies are due to unsafe acts
(behaviours) by people. For example, improper equipment use, not following
procedures, positions / reactions of people, housekeeping. But, rather than
being the instigators, it is typically the case that unsafe (organisational)
conditions, that have been long developing and that have been inherited by
people, represent the root cause(s) of accidents and incidents. Examples of
systemic organisational weaknesses include lack of supervision, ill-defined
roles and responsibilities, inadequate training / assessment / procedures /
instructions, poor leadership and safety communications, competing job demands,
ineffective planning and safe systems of work. Such examples obviously
correspond to symptoms of a poor or negative safety culture.
In an organisation, the benefit in
consulting, sharing information and giving workers reasonable opportunity to
express their views and contribute towards decision making on health and safety
matters is that it can assist the employer in meeting its health and safety
obligations, thus more profit resulting from preventive measures of related
risks.
Through the research it has been revealed that
questionnaires have a wide area of application. It can be used within an
organisation for diagnosing the organisation's safety culture level, and
bringing into focus how the organisation handles safety cultural issues. It can
be used as a personal assessment tool which can create motivation and awareness
for safety culture among organisational members (Camilla, 2003).
This research work is made up of four
chapters. Chapter I gives some brief presentation and activities of
LABORATOIRES BIOPHARMA S.A. Chapter II places our study in the context of the
literatures concerning OHSAS 18001:2007 with much emphasis on safety cultures
and the Cameroonian law on health and safety at workplace (Order No
039/MTPS/IMT of 26 November 1984). Chapter III outlines and spells out the
materials and methodology used, with explanations of why and how it was used to
yield our results, their interpretation, discussions and the recommendations
expressed in chapter IV. Finally the general conclusion will close up our
research.
CHAPTER I: PRESENTATION OF
COMPANY
In the paragraph below, we shall
write briefly on the presentation, activities and administrative structure of
BIOPHARMA S.A
«LES LABORATOIRES BIOPHARMA S.A» whose short form is
BIOPHARMA in our memoire, was created on the 04th of July 2001. It
is a private company with a current capital of 700,000,000 FCFA headed by a
General Manager named Mr DJOMOU NANA Francis. Biopharma employs over 180
permanent workers within its two production units. The first is the plastic
unit which produces most of our plastic bottles, caps and nylon films meanwhile
the second unit is meant for the manufacturing of cosmetic products. The main
activity of Biopharma is the production and distribution of cosmetic products.
More details are shown in table 1 and Figure 1 below.
LES LABORATOIRES BIOPHARMA S.A (Figure 1) found in Cameroon is
located in the Littoral region, Wouri division, Douala 3 subdivision at the
Magzi industrial zone Bassa. With the GPS (Figure 2), datae were collected and
used to indicate the precise position of our company as shown in the map below
(Figure 3).
 
Figure 1: Main entrance of Biopharma. Figure
2: GPS collecting datae
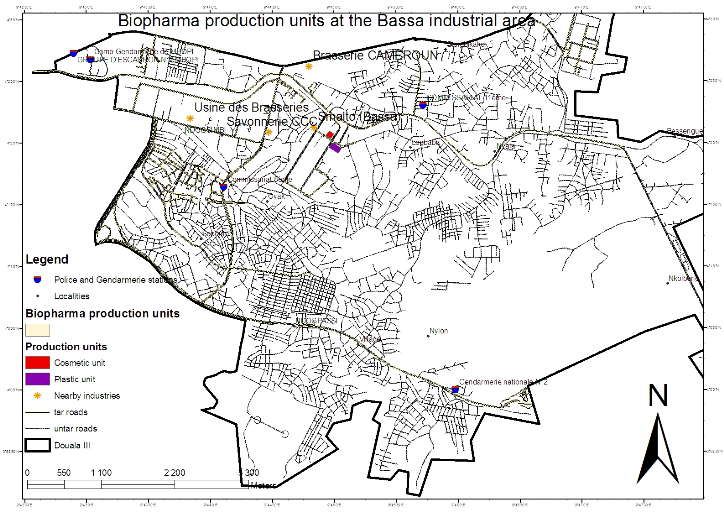
Figure 3: Map for the localisation of Biopharma S.A.
Table 1: Presentation and Activities of Biopharma
S.A
|
Identity of firm
|
|
|
Name of firm
|
LES LABORATOIRES BIOPHARMA S.A.
|
|
Address
|
P.O BOX 1674 Douala magzi industrial zone Bassa /
Cameroon
|
|
Contacts (IT)
|
Telephones
|
(00237) 33 37 56 16
(00237) 77 93 44 87
|
|
E-mail
|
biopharma@camnet.cm
|
|
General Manager
|
Mr Francis DJOMOU NANA
|
|
Created on
|
04 Juillet 2001 (1st production : 2002)
|
|
Activities
|
Principal
|
Production and distribution of cosmetic and OTC products.
|
|
Secondary
|
Plastic unit which produces our plastic bottles, caps and
films (shrink nylon)
|
|
Type of Company
|
PRIVATE PLC
|
|
Tax payer statistic No
|
M070100012311D
|
|
|
Business register No
|
026968
|
|
|
Financial situation
|
Turn-over
|
700 000 000 FCFA
|
|
Principal brands
|
Primo, Rapid'clair, Essentiel, Stay White, Talangai, Lumina,
Pluviherbal, Pluvicare, White Express Moby bébé, Nivys, Bettina,
Citro clair, Biosuccess, BLIGHT, Talcum powder, Parfumes, Nail polish (Celine
France, Lumina) etc.
|
|
Localisation of Customers
|
Cameroon, Gabon, Democratic Republic of Congo, Angola, RCA,
West Africa, Europe (Italy, Spain)
|
|
Competitors
|
SIPCA, GANDOUR, Products imitated in Nigeria
|
|
Suppliers of licenced products
|
Mirato - Italy, Evoluderm and Dark § Lovely - France
|
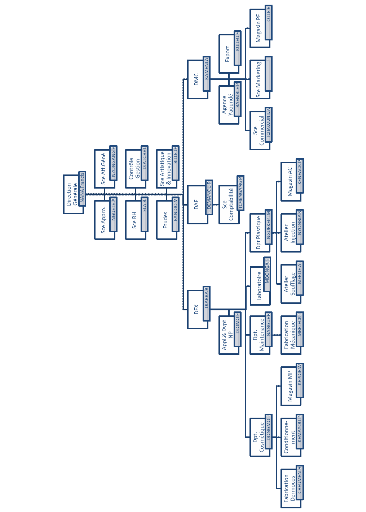
Figure 4:
Organigram of Biopharma 2012
| 


