CHAPTER II: LITERATURE
REVIEW
I. GENERAL OVERVIEW
A. DEFINITION OF TERMS
· Adherence (to a medication
regimen): The World Health Organization (WHO) defines adherence as ``the extent
to which a person's behaviour taking medication, following a diet, and/or
executing life style changes corresponds with agreed recommendations from a
health care provider''[23].
· Disability-Adjusted Life Year
(DALY):Measure of years of life lost from deaths which occur before
some theoretically achievable age (e.g., international reports use 80 years for
men and 82.5 years for women) and attributing this loss to death rates. DALYs
for a disease or health condition are calculated as the sum of the Years of
Life Lost due to premature mortality in the population and the Years Lost due
to Disability for people living with the health condition or its
consequences[24].
· Compliance: Term suggesting that a
patient is passively following the doctor's orders and that the treatment plan
is not based on a therapeutic alliance or contract established between the
patient and the physician[10].
· Hypertension:Systolic blood pressure
equal to or above 140 mm Hg and/or diastolic blood pressure equal to or above
90 mm Hg[1].
B. EPIDEMIOLOGY OF HYPERTENSION
In 2010, the three leading risk factors for global disease
burden were high bloodpressure (7.0% of global DALYs), tobacco smoking
includingsecond-hand smoke (6.3%), and alcohol use (5.5%)[2].
Hypertension alone accounts for 9.4 million deaths
worldwide[1].Dietaryrisk factors and physical inactivity
collectively accounted for 10.0% of globalDALYs in 2010, with the most
prominent dietary risks being diets low in fruits and those high
insodium[2].Hypertension is responsible for at least 45% of
deaths due toheart disease, and 51% of deaths due
tostroke[1].Figure 1 shows the total stroke mortality rate in
the world.Globally, the overall prevalence of raised blood pressure in adults
aged 25 and over was around 40% in 2008[4].Figure 2 shows the
global age-standardized prevalence of raised blood pressure in adults aged 25+
years.The proportion of the world's population with high blood pressure, or
uncontrolled hypertension, fell modestly between 1980 and 2008. However,
because of population growth and ageing, the number of people with hypertension
rose from 600 million in 1980 to nearly 1 billion in 2008[4].
Still in 2008, the prevalence of raised blood pressure was highest in the
African continent, where it was 46% for both sexes
combined[4].

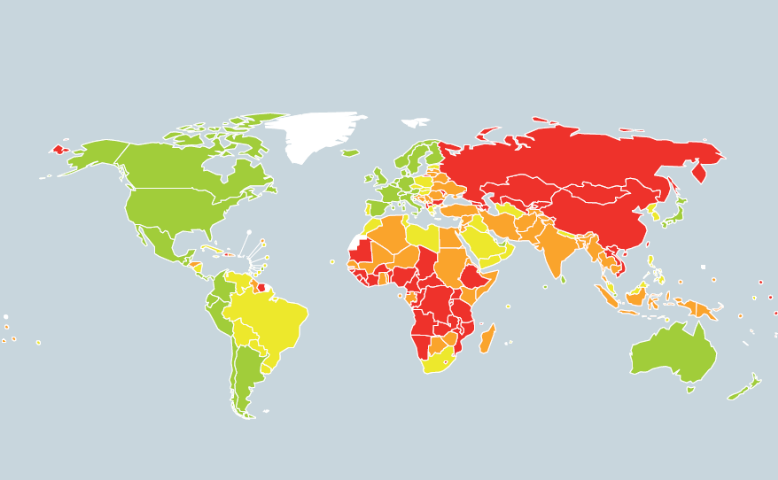
Figure 1: Cerebrovascular disease mortality
rates[1]
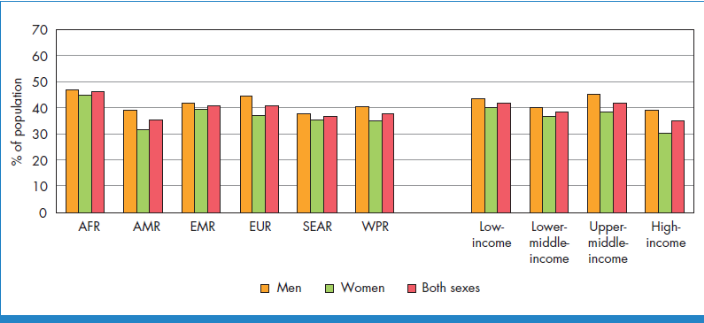
Figure 2: Age-standardized
prevalence of raised blood pressure in adults aged 25+ years[4]
The lowest prevalence of raised blood pressure was in the WHO
Region of theAmericas, with 35% for both sexes[4]. In all WHO
regions, men have slightly higher prevalence of raised blood pressure than
women, but this difference was only statistically significant in the American
and European continents[4].Across the income groups of
countries, the prevalence of raised blood pressure was consistently high in low
and lower-middle income countries while upper-middle-income countries all had
rates of around 40%for both sexes. The prevalence in high-income countries was
lower, at 35% for both sexes[4].Not only is hypertension more
prevalent in low- and middle-income countries, there are also more people
affected because more people live in those countries than in high-income
countries[1,5].Further, because of weak health systems, the
number of people with hypertension who are undiagnosed, untreated and
uncontrolled are also higher in low- and middle-income countries compared to
high-income countries. The increasing prevalence of hypertension is attributed
to population growth, ageing and behavioural risk
factors[1,11].Figure 3 shows the 2015 distribution of the
world's population by age and sex.
The adverse health consequences of hypertensionare compounded
because many peopleaffected also have other health risk factorsthat increase
the odds of heart attack,stroke and kidney failure. These risk factorsinclude
tobacco use, obesity, high cholesteroland
diabetesmellitus[1].In 2008, 1 billion people weresmokers and
the global prevalence of obesity has nearly doubled since
1980[1]. The global prevalenceof high cholesterol was 39% and
prevalenceof diabetes was 10% in adults over 25years[4].
Tobacco use, unhealthy diet, harmfuluse of alcohol and physical inactivity are
alsothe main behavioural risk factors of all majornoncommunicable diseases,
i.e. cardiovasculardisease, diabetes, chronic respiratory disease and cancer.If
appropriate action[1] is not taken, deaths dueto
cardiovascular disease are projected to rise further
[25].Figure 4 shows the projected global deaths for selected
causes for the 2004-2030 timeframe.
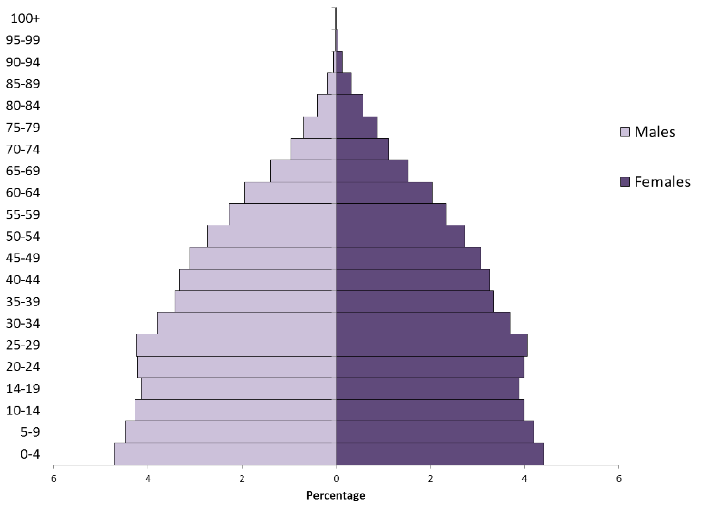
Figure 3: Distribution of the
world's population by age and sex, 2015[11]
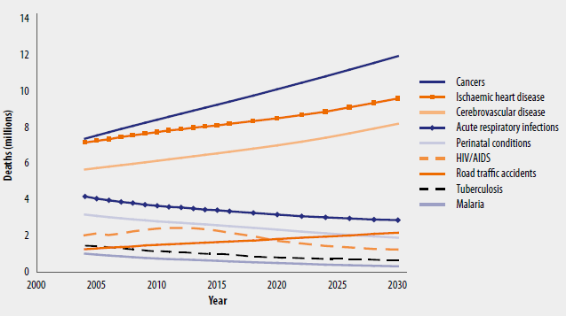
Figure 4: Projected global
deaths for selected causes, 2004-2030[25]
Between 1994 and 2003, blood pressure levels havedeteriorated
over time in rural and urban Cameroonian menand women with the prevalence of
hypertension increasing by twofold to fivefold[26].This has
beenattributed to the rapid urbanization associated with thehigh rates of
obesity, physical inactivity, diabetes,increased salt consumption, and tobacco
use[27].In 2011, Dzudie A et al. reported a prevalence rate
for hypertension of up to 47.5% in self-selectedurban dwellers in
Cameroon[28].In a more recent nationwide study, Kingue et al.
found a prevalence rate of 29.7% in urban areas of Cameroon;indicating
asteadyrise in the trend of hypertension toward a super epidemic in 20 years to
come[8].
C. CAUSES OF HYPERTENSION
In the majority of cases, high BP is termedprimary or
essentialhypertensionand isprobably multifactorial in origin, with genotype, as
well asexternal factors such as diet and body-weight, playing arole.
Hypertension may also be associated with surgery[29]or
pregnancy[30] and is prevalent in
diabetics[31]. In a limitednumber of cases hypertension is
secondaryto some othercondition, such as renal disease, Cushing's
syndrome,phaeochromocytoma, or to the adverse effects of drugs suchas
oestrogens, and such causes may be suspected particularlyin resistant or
malignant hypertension[32].
1. Primary or essential
hypertension[1,33,34]
Although it has frequently been indicated that the causes
ofessential hypertension are unknown, this is only partially true because
little information is available on genetic variationsor genes that are
over-expressed or under-expressed as well asthe intermediary phenotypes that
they regulate to cause hypertension.Variation in BP that is genetically
determined is called «inherited BP,»although the genes which cause BP
to vary are not known; it is known from familystudies that inherited BP can
range from low normal BP tosevere hypertension. Factors that increase BP, (such
as obesity, insulin resistance, high alcohol intake, high salt intake (in
salt-sensitive patients), ageing and perhapssedentary lifestyle, stress, low
potassium intake, and low calcium intake) are called «hypertensinogenic
factors.» Some of these factors have inherited,behavioural, and
environmental components. Inherited BPcould be considered core BP, whereas
hypertensinogenicfactors cause BP to increase above the range of inherited BPs.
Figure 5 illustrates the additive effect of hypertensinogenic factors on
hereditary systolic and diastolic BP. It shows that patients with normalor high
normal inherited BP become hypertensive stage 1 whenBP is increased by a
hypertensinogenic factor. In patients withinherited hypertension in stages 1 to
3, their hypertensionbecomes more severe when hypertensinogenic factors
areadded.
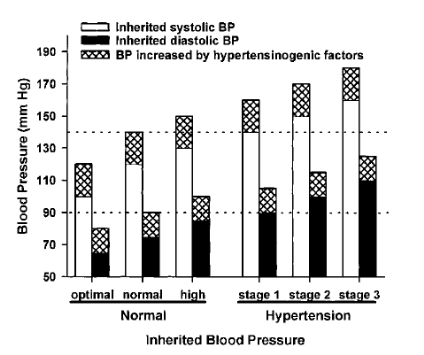
Figure 5: Additive effect
of hypertensinogenic factors (hatched areas) on hereditary systolic (white
areas) and diastolic blood pressure (black areas)[33]
2. Secondary hypertension[32,35,36]
Hypertension related to a specific aetiology is termed
secondaryhypertension,markedly differing from essential hypertension, ofwhich
the aetiology cannot be clearly identified. Secondary hypertension is often
resistant hypertension,for which a target blood pressure is difficult to
achieve bystandard treatment. However, BP can be effectivelyreduced by
identifying its aetiology and treating the condition.
Frequent etiological factors for secondary hypertension
includerenal parenchymal hypertension, primary aldosteronism (PA),
renovascularhypertension and sleep apnea syndrome. As other etiological factors
for secondary hypertension, the following conditions have been reported in
endocrine hypertension: pheochromocytomaand Cushing's syndrome which are
related to an excessiveproduction of catecholamines and cortisol, respectively.
Hypo-/hyperthyroidism, hyperparathyroidism and acromegaly are alsoetiologically
involved in hypertension. In vascular hypertension, angiitissyndrome, such as
aortitis syndrome, polyarteritis nodosa (PN)and systemic scleroderma, aortic
coarctation and aortic insufficiencyhave been reported. Compression of the
rostral ventrolateral medullaby brainstem blood vessels causes hypertension
through hyperactivityof the sympathetic nerves. Furthermore, hypertension is
also observedin patients with brain tumours or cerebrovascular disease. In
addition,drug-induced hypertension has been reported (by drugs such as NSAIDs,
oral contraceptive, liquorice, sympathomimetics, glucocorticoids,
cyclosporine, tacrolimus, erythropoietin, tricyclic/tetracyclic
antidepressants, monoamine oxygenase inhibitors, Anti-VEGF antibody
preparations).
It has been recognized that secondary hypertension accounts
for about 10% of hypertensive patients. According to several studies, PA
accounts for approximately 5-10% of hypertensive patients, and it is the most
frequent in endocrine hypertension.
Generally, the presence of severe or resistant hypertension,
juvenile hypertension and the rapid onset of hypertension suggest the
possibility of secondary hypertension. In such hypertensive patients, a close
inquiry on medical history, medical examination and adequate examinations must
be performed, considering the possibility of secondary hypertension. The
possibility of secondary hypertension should be considered in the diagnosis and
treatment of all hypertensive patients. It is important to conduct appropriate
examinations without overlooking findings of secondary hypertension.
D. THE SYMPTOMS OF HIGH BP AND ITS
COMPLICATIONS[1,37,38]
There is a common misconceptionthat people with
hypertensionalwaysexperience symptoms, but the reality isthat mosthypertensive
people have nosymptoms at all. The condition is a silent killer. Therefore it
is important for everybody to know their blood pressure reading.
Sometimes hypertensioncauses symptoms such as headache,
shortnessof breath, dizziness, chest pain, palpitationsof the heart and nose
bleeds. It canbe dangerous to ignore such symptoms,but neither can they be
relied upon to signifyhypertension.
Left untreated, high BP can have damaging effects. Theprimary
way it causes harm is by increasing the workload of the heart and
arteries,which causes damage to the circulatory system over time. Also, high BP
can cause the heart to enlarge because it has to work harderto supply the blood
the body needs. It is also a contributing factor toatherosclerosis, in which
the walls of the arteries become stiff and brittle as fattydeposits build up
inside them. Other conditions caused by hypertension include coronary heart
disease, heart failure, heartattack, stroke, kidney damage, angina (chest pain
related to heart disease), peripheralartery disease, and other serious
conditions (aneurysms, cognitive changes, eye damage). In fact, people with BP
over 140/90 are far more likely to havethese dangerous conditions thus
hypertension is a serious warning sign that significant lifestyle changes have
to be adopted.
E. DIAGNOSTIC EVALUATION
Current options for BP measuring devices include mercury
sphygmomanometers, aneroid manometers, semiautomatic devices and fully
automatic electronic devices. Validated and affordable electronic BP measuring
devices, that have the option to select manual readings, appear to be the
preferred option for lowresource settings according to
WHO[39].Semi-automatic devices enable manual readingsto be
taken when batteries run down,a common problem in resource-constrainedsettings.
Given that mercury is toxic,it is recommended that mercury devices bephased out
in favour of electronic devices[39].Aneroid devices should be
considered only if calibrated at regular intervals (every 6 months for example)
and users should be trained and assessed in measuring BP using such
devices[1,39].
Diagnostic procedures aim at: 1) establishing blood
pressurelevels; 2) identifying secondary causes of hypertension;3) evaluating
the overall cardiovascular risk bysearching for other risk factors, target
organ damageand concomitant diseases or accompanying clinicalconditions.The
diagnostic procedures comprise[40]:
- repeated blood pressure measurements
- medical history
- physical examination
- laboratory and instrumental investigations.
Some ofthese should be considered part of the routine
approachin all subjects with high BP; some arerecommended and may be used
extensively in thedeveloped health systems; some are indicatedonly when
suggested by the basic examination or theclinical course of the
patient[40].
1. Blood pressure measurement[1,40-42]
Blood pressure is characterized by large spontaneousvariations
both during the day and between days, monthsand seasons.Therefore the diagnosis
of hypertension should be based on multiple BP measurements, taken on separate
occasions over a period of time. If BP is only slightly elevated, repeated
measurements should be obtained over a period of several months to define the
patients ``usual'' BP as accurately as possible. On the other hand, if the
patient has a more marked BP elevation, evidence of hypertension-related organ
damage or a high or very high cardiovascular risk profile, repeated
measurements should be obtained over shorter periods of time (weeks or days).
In general, the diagnosis of hypertension should be based on at least 2 blood
pressure measurements per visit and at least 2 to 3 visits, although in
particularly severe cases the diagnosis can be based on measurements taken at a
single visit. Blood pressures can be measured by the doctor or the nurse in the
office or in the clinic (office or clinic blood pressure), by the patient or a
relative at home, or automatically over 24 h. Based on specific recommendations
of the European Society of Hypertension[40], these procedures
can be summarized as follows:
1.1 Office or clinic blood pressure
BP can be measured by a mercury sphygmomanometer the various
parts of which (rubber tubes, valves, quantity of mercury, etc.) should be kept
in proper working order. Other non-invasive devices (auscultatory or
oscillometric semiautomatic devices) can also be used and will indeed become
increasingly important because of the progressive banning of the medical use of
mercury[39]. However, these devices should be validated
according to standardized protocols[43], and their accuracy
shouldbe checked periodically by comparison with mercurysphygmomanometric
values. Table I shows the instructions for correctoffice BP measurements.
1.2 Ambulatory blood
pressure
Several devices (mostly oscillometric) are available
forautomatic BP measurements in patients allowed to conduct a near normal life.
They provide information on 24-hour average BP as well as on mean values over
more restricted periods such as the day, night or morning. This information
should not be regarded as a substitute for information derived from
conventional BP measurements. Studies have shown that ambulatory BP : 1)
correlates with hypertension-related organ damage and it changes by treatment
more closely than does office blood pressure, 2) has a relationship with
cardiovascular events that is steeper than that observed for clinic BP, with a
prediction of cardiovascular risk greater than the prediction provided by
office BP values in populations of untreated and treated
hypertensives[44-46] and 3) measures more accurately than
clinic BP the extent of BP reduction induced by treatment, because of a higher
reproducibility over time and an absent or negligible ``white
coat''[47] and placebo effect[48].
Table I: Blood pressure (BP)
measurement[40]
|
When measuring BP, care should be taken to:
|
|
1
|
Allow the patients to sit for several minutes in aquiet room
before beginning BP measurements
|
|
2
|
Take at least two measurements spaced by1-2 minutes, and
additional measurements if thefirst two are quite different
|
|
3
|
Use a standard bladder (12-13 cm long and 35 cmwide) but have a
larger and a smaller bladderavailable for fat and thin arms, respectively.
Usethe smaller bladder in children
|
|
4
|
Have the cuff at the heart level, whatever theposition of the
patient
|
|
5
|
Use phase I and V (disappearance) Korotkoffsounds to identify
systolic and diastolic BP,respectively
|
|
6
|
Measure BP in both arms at first visit to detectpossible
differences due to peripheral vasculardisease. In this instance, take the
higher valueas the reference one
|
|
7
|
Measure BP 1 and 5min after assumption ofthe standing position in
elderly subjects, diabeticpatients, and in other conditions in which
posturalhypotension may be frequent or suspected
|
|
8
|
Measure heart rate by pulse palpation (at least30 sec) after the
second measurement in the sittingposition
|
Although some of the aboveadvantages can be obtained by
increasing the number ofoffice BP measurements, 24-hourambulatory BP monitoring
may be useful atthe time of diagnosis and at varying intervals
duringtreatment[40]. Efforts should be made to extend
ambulatory BP monitoring to 24 hours in order to obtaininformation on both
daytimeand night-timeBP profiles, day-night BP difference, morning BP rise and
BP variability.Daytimeand night-time blood pressure values and changes
bytreatment are related to each other, but the prognosticvalue of night-time
blood pressure has been found tobe superior to that of daytime blood
pressure[45,49]. Evidence is also available thatcardiac and
cerebrovascular events have a peak prevalencein the morning,possibly in
relation to thesharp blood pressure rise occurring at awaking fromsleep, as
well as to an increased plateletaggregability, a reduced fibrinolytic activity
and a sympatheticactivation[40,41].
When measuring 24-hour blood pressure[50]
care shouldbe taken to:
· Use only devices validated by international
standardized protocols
· Use cuffs of appropriate size and compare the initial
values with those from a sphygmomanometer to checkthat the differences are not
greater than #177; 5mmHg
· Set the automatic readings at no more than 30 min
intervals to obtain an adequate number of values and have most hours
represented if some readings arerejected because of artefact.
· Automatic deflation of the equipment should be at arate
of no more than 2mmHg/s.
· Instruct the patients to engage in normal activities
but to refrain from strenuous exercise, and to keep the armextended and still
at the time of cuff inflations.
· Ask the patient to provide information in a diary on
unusual events and on duration and quality of nightsleep.
· Obtain another ambulatory BP if the first examination
has less than 70% of the expected number of valid values because of frequent
artefacts. Ensure that the proportion of valid values is similar for the dayand
night periods.
· Remember that ambulatory BP is usually several mmHg
lower than office BP. Different population studies indicate that office values
of 140/90mmHg correspond to average 24-h values of either 125-130mmHg systolic
and 80mmHg diastolic, the corresponding average daytime and night-time values
being 130-135/85 and 120/70mmHg. These values may be regarded as approximate
threshold values for diagnosinghypertension by ambulatory BP. Table II
indicates blood pressure thresholds for the definition of hypertension with
different types of measurement
· Clinical judgement should be mainly based on average
24-hour, day and/or night values. Other information derived from ambulatory
blood pressure (e.g. morning blood pressure surge and blood pressure standard
deviations) is clinically promising, but the field shouldstill be regarded as
in the research phase.
Table II: Blood pressure
thresholds (mmHg) for definition of hypertension with different types of
measurement[40]
|
SBP
|
DBP
|
|
Office or clinic
|
140
|
90
|
|
24-hour
|
125-130
|
80
|
|
Day
|
130-135
|
85
|
|
Night
|
120
|
70
|
|
Home
|
130-135
|
85
|
2. Family and clinical history[40]
A comprehensive family history should be obtained
withparticular attention to hypertension, diabetes, dyslipidaemia,premature
coronary heart disease, stroke, peripheralartery or renal disease.The clinical
history should include: a) duration andprevious levels of high blood pressure;
b) symptomssuggestive of secondary causes of hypertension andintake of drugs or
substances that can raise blood pressure,such as liquorice, nasal drops,
cocaine, amphetamines,oral contraceptives, steroids, nonsteroidal
anti-inflammatory drugs, erythropoietin, and cyclosporin;c) lifestyle factors,
such as dietary intake of fat (animalfat in particular), salt and alcohol,
quantification of smokingand physical activity, weight gain since early
adultlife; d) past history or current symptoms of coronarydisease, heart
failure, cerebrovascular or peripheralvascular disease, renal disease, diabetes
mellitus, gout,dyslipidaemia, asthma or any other significant illnesses,and
drugs used to treat those conditions; e) previousantihypertensive therapy, its
results and adverse effects;and f) personal, family and environmental factors
thatmay influence blood pressure, cardiovascular risk, as wellas the course and
outcome of therapy. Also, physiciansshould enquire after the patient and/or
partner aboutsnoring which may be a sign of sleep apnoea syndromeand increased
cardiovascular risk.
3. Physical examination
In addition to BP, heart rate should be carefullymeasured
(pulse counting over at least 30s or longer ifarrhythmias are reported) because
the repeated findingof values above normal may be an indication of greaterrisk,
increased sympathetic or decreased
parasympatheticactivity[51], or of heart failure. Physical
examinationshould search for evidence of additional risk factors, forsigns
suggesting secondary hypertension, and for evidenceof organdamage. Table III
highlights the physical examination for secondary hypertension, organ damage
and visceral obesity.Waist circumference should be measuredwith the patient
standing and body weight and heightshould be obtained to calculate the body
mass index.
4. Laboratory investigations
Laboratory tests are directed at providing evidencefor
additional risk factors, searching for secondaryhypertension and looking for
the absence or presenceof organ damage. Investigations should progress from
themost simple to the more complicated. The youngerthe patient, the higher the
BP and the fasterthe development of hypertension, the more detailed
thediagnostic work-up should be. However, the minimum laboratory investigations
needed remain a matter ofdebate.
Table III: Physical
examination for secondary hypertension, organ damage and visceral
obesity[40]
|
Signs suggesting secondary hypertension and
organ damage
|
-Features of Cushing syndrome
-Skin stigmata of neurofibromatosis(phaeochromocytoma)
-Palpation of enlarged kidneys (polycystic kidney)
-Auscultation of abdominal murmurs (renovascular
hypertension)
-Auscultation of precordial or chest murmurs (aortic
coarctation or aortic disease)
-Diminished and delayed femoral pulses and
reduced femoral BP (aortic coarctation, aortic
disease)
|
|
Signs of organ damage
|
-Brain: murmurs over neck arteries, motor or
sensory defects
-Retina: fundoscopic abnormalities
-Heart: location and characteristics of apical
impulse, abnormal cardiac rhythms, ventricular
gallop, pulmonary rales, peripheral oedema
-Peripheral arteries: absence, reduction, or asymmetryof pulses,
cold extremities, ischaemic skinlesions
-Carotid arteries: systolic murmurs
|
|
Evidence of visceral obesity
|
-Body weight
-Increased waist circumference (standing position)
M: > 102 cm; F: > 88 cm
-Increased body mass index [body weight (kg)/
height (m)2]
-Overweight =25 kg/m2; Obesity =30
kg/m2
|
Where cardiovasculardiseases are the primary cause of
morbidityand mortality, routine laboratory investigations shouldinclude: blood
chemistry for fasting glucose, total cholesterol,LDL-cholesterol,
HDL-cholesterol, triglycerides(fasting), urate, creatinine, potassium,
haemoglobin andhaematocrit; urinalysis by a dipstick test that permits
thedetection of microalbuminuria; urine microscopic examinationand an
electrocardiogram[40].
Serum creatinine values should be used to estimatecreatinine
clearance via the Cockroft Gault formula[52], easy procedures
allowing identificationof patients with reduced glomerular filtration
andincreased cardiovascular risk but in whom serum creatininevalues are still
in the normal range. When fasting plasma glucose is =5.6 mmol/L(100 mg/dL), a
post-load plasma glucose (glucose tolerancetest) is
recommended[53]. The repeated findingof a fasting plasma
glucose =7.0 mmol/L (126 mg/dL),and an abnormal glucose tolerance test are
consideredindicative of diabetes mellitus[53].
Although highsensitivity C reactive protein (hsCRP)has been
reportedto predict the incidence of cardiovascular events inseveral clinical
settings[54], its added value in determiningtotal
cardiovascular risk is uncertain,except in patients with metabolic syndrome in
whomhsCRP values have been reported to be associatedwith a further marked
increase in risk[55].Table IV gives a summary of the possible
laboratory investigations.
Table IV: Laboratory
investigations[40]
|
Routine tests
|
_ Fasting plasma glucose
_ Serum total cholesterol
_ Serum LDL-cholesterol
_ Serum HDL-cholesterol
_ Fasting serum triglycerides
_ Serum potassium
_ Serum uric acid
_ Serum creatinine
_ Estimated creatinine clearance (Cockroft-Gaultformula) or
glomerular filtration rate (MDRDformula)
_ Haemoglobin and haematocrit
_ Urinalysis (complemented by microalbuminuriavia dipstick test
and microscopic examination)
_ Electrocardiogram
|
|
Recommended tests
|
_ Echocardiogram
_ Carotid ultrasound
_ Quantitative proteinuria (if dipstick test positive)
_ Ankle-brachial BP Index
_ Fundoscopy
_ Glucose tolerance test (if fasting plasma glucose>5.6 mmol/L
(100 mg/dL)
_ Home and 24 h ambulatory BP monitoring
_ Pulse wave velocity measurement (where available)
|
F. MANAGEMENT OF HYPERTENSION
Most of what follows is linked to primary or essential
hypertension in adults.Hypertension may be discovered because of adverse
vascularevents, especially in the eyes, brain, kidneys, orheart, but is more
often asymptomatic and only discoveredon routine measurement of blood
pressure[1,38]. Once diagnosed,decisions have to be made about
the need for treatment.It is well-established that hypertension is a risk
factorfor the development of stroke, heart failure, and renaldamage, and to a
lesser extent ischaemic heart disease, anda reduction in blood pressure is
generally beneficial, althoughmortality remains higher than in
non-hypertensives[56].
Treatment of hypertensionmay involve both non-pharmacological
and pharmacologicalinterventions to reduce blood pressure, as well asassessment
and treatment of any other cardiovascular riskfactors; anyco-existing diseases
should also be treated[3]. Differences inthe detail of
guidelines on the management of hypertensionreflect varying judgements on the
justification for interventionand the relative risks and benefits of
differenttreatments.
1. Non-pharmacological treatment
Adopting a healthylifestyle is beneficial for all individuals,
and any patientwith raised blood pressure should be encouraged to makelifestyle
changes that will reduce their cardiovascular risk.Some of these changes may
also reduce blood pressure,and in those who are at low overall risk no other
treatment may be needed[57,58]. A trial of
non-pharmacologicaltreatment is recommended in most patients beforestarting
drug therapy, but should not unnecessarily delaytreatment, especially if the
patient is at high risk[40,59-61]. Interventionsthat have been
shown to reduce blood pressureinclude[1,3]:
Ø reduction in excess weight
Ø reduction in excess alcohol consumption
Ø reduction in sodium intake
Ø adequate exercise
Ø reduced fat intake
Ø increased fruit and vegetable consumption
Other interventions that have been tried, but with less
evidenceof benefit, include[3]:
Ø increased intake of potassium, magnesium, and
calcium
Ø increased polyunsaturated fat intake with reduced
saturated fat intake
Ø relaxation therapies for stress reduction.
These lifestyle changes may also be promoted in the
populationas a whole, or in individuals most likely to develophypertension, in
strategies for the primary preventionofhigh blood
pressure[1,62].
2. Pharmacological treatment
The main decisions in drugtreatment relate to the blood
pressure at which therapyshould be begun, the target blood pressure, and the
mostappropriate drug regimen to use[3]. Controversies exist in
allthese areas.When to intervenewith antihypertensive drugs dependson factors
including both the measured blood pressure andthe overall cardiovascular risk.
Several guidelines have been published on the measures which entail
pharmacological treatment[40,59,60]:
· Patients with grade 3 hypertension (180/110 mmHg or
higher) should receive prompt drug treatment.
· In grade 2 hypertension, drug therapy is indicated if
blood pressure remains at 160/100 mmHg or higher after a period of lifestyle
modification, which varies depending on the overall level of risk; prompt drug
therapy is advised for those at high or very high risk.
· For patients with grade 1 hypertension, the need for
treatment is less well established; those with associated risk factors should
be given drug therapy if lifestyle modification is inadequate, but some
guidelines suggest that antihypertensives are not indicated in those at lower
risk, or state that priority should be given to those at highest risk.
· Lower thresholds may apply in patients with renal
disease or diabetes, but whether there is any benefit in treating uncomplicated
patients with prehypertension is controversial.
Guidelines therefore generallyrecommend that treatment
decisions should not be basedon age, although slower titration of drugs has
beensuggested[40] in older patients since they may be more
susceptibleto adverse effects. In the very old (those over 80years) the benefit
of starting therapy is less clear[63,64], althougha
study[65] in patients aged 80 years and over found a reduction
in mortality. Those already being treated
shouldcontinue[40,60].
Target blood pressuresare also controversial. The HOT study
found that effective control to maintain the diastolic pressure at about 85
mmHg reduced therate of cardiovascular events, but lower pressures (ofaround 70
mmHg) did not provide any further benefit[66]. Target blood
pressures of below140/90 mmHg[40,61], or below 140/85
mmHg[60] are now recommended;lower targets may be considered
if toleratedby the patient, particularly in patients at high
risk[40]. A lowertarget of below 130/80 mmHg has also been
suggested for patients with established ischaemic heart
disease[67], andlower targets may also be appropriate in
diabetics and patients with renal disease.
Antihypertensive drugs comprise several classes of active
pharmaceutical ingredients (APIs) with the therapeutic objective of controlling
hypertension. Table V shows the different classes and subclasses of
antihypertensives.
Table V: Classes and
subclasses of antihypertensive medications with common examples[68]
|
CLASS
|
EXAMPLES
|
|
Targeting renin-angiotensin system
|
|
Angiotensin-converting
enzyme inhibitors
|
Captopril, lisinopril, ramipril
|
|
Angiotensin receptor
antagonists
|
Candesartan, losartan, valsartan
|
|
Direct renin antagonists
|
Aliskiren
|
|
Adrenoceptor antagonists
|
|
â-Blockers
|
Atenolol, metoprolol, propranolol
|
|
á-Blockers
|
Doxazosin, labetalol (also a â-blocker),
phentolamine, phenoxybenzamine
|
|
Calcium channel blockers
|
|
Phenylalkylamine
|
Verapamil
|
|
Dihydropyridines
|
Amlodipine, nifedipine, nimodipine
|
|
Benzothiazepines
|
Diltiazem
|
|
Diuretics
|
|
Thiazides
|
Bendroflumethiazide,
hydrochlorothiazide
|
|
Loop
|
Furosemide, bumetanide
|
|
Potassium sparing/
aldosterone antagonist
|
Amiloride, spironolactone
|
|
Vasodilators
|
Hydralazine, minoxidil
|
|
Centrally acting agents
|
Clonidine, methyldopa
|
|
Ganglion block
|
Trimetaphan
|
The classes of antihypertensive drugs differ in their chemical
structures thence their varying functions. Antihypertensive drugs are
frequently used in other unrelated conditions, for
example, â-blockers
in thyrotoxicosis[69]and anxiety[70], or
angiotensin-converting enzyme inhibitors (ACEIs) in heart failure.
The following subsections will focus on the applied
pharmacology of agents used in the management of hypertension, some of their
side-effects, as well as some drug interactions.
o Drugs which target the renin-angiotensin system
(RAS)
Three drug classes directly target points of the RAS pathway.
They act to reduce production of the peptide hormone angiotensin II, or reduce
its receptor binding. Figure 6 shows the different sites of action of drugs
affecting the renin-angiotensin system.
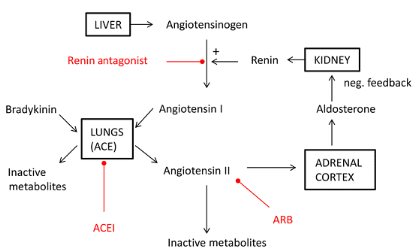
Figure 6: Sites of action
of drugs affecting the renin-angiotensin system.[68]
Angiotensin II has high affinity for AT1 G-protein-coupled
receptors, activation of which causes increased arteriolar tone and systemic
vascular resistance (SVR). It also causes sympathetic nervous system
activation, increased pituitary secretion of antidiuretic and
adrenocortocotrophic hormones, and increased adrenocortical secretion of
aldosterone[71].By antagonizing the RAS pathway, SVR and
arterial pressure are reduced. This effect is potentiated by a reduction in
aldosterone secretion with resultant reduction in renal sodium and water
retention. Negative feedback results in increased renin release by the
juxtaglomerular apparatus.
1. ACEI drugs[3,68,71-73]
The discovery that the venom of the Brazilian pit viper, which
causes a massive decrease in arterial pressure, works by inhibition of
angiotensin-converting enzyme (ACE) led to the development of synthetic, orally
administered ACEIs. ACEIs are among the first-line treatment in non-black
patients under 55 years of age with primary hypertension.They are also
indicated in heart failure, post-myocardial infarction, diabetic nephropathy,
and chronic kidney disease (although not acute kidney injury). The renaland
cardiac protective effects of ACEIs are greater than those expected by arterial
pressure control alone.
ACE is a metallopeptidase enzyme which occurs mainly within
the pulmonary vasculature. The inhibition of ACE reduces the cleavage of the
decapeptide hormone angiotensin I to the octapeptide angiotensin IIand reduces
metabolism of the peptide bradykinin to inactivesubstances. The reduction in
angiotensin II is responsible formost of the therapeutic effects.
v Perindopril
o Chemical structure
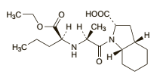 C19H32N2O5 C19H32N2O5
o Pharmaceutical form and administration
Perindopril is available as oral tablets and when taken as the
erbumine salt should be taken before food. Perindopril is also available as the
arginine salt; 5 mg ofperindopril arginine is equivalent to about 4 mg
ofperindopril erbumine.In the treatment of hypertension perindopril is givenin
an initial dose of 4 mg of the erbumine or 5 mg of thearginine salt once
daily.
o Pharmacokinetic data
Perindopril acts as a prodrug of the diacid perindoprilat, its
active form. After oral doses perindopril is rapidly absorbed with a
bioavailability of about 65 to 75%. It is extensively metabolised, mainly in
the liver,to perindoprilat and inactive metabolites including glucuronides. The
presence of food is reported to reducethe conversion of perindopril to
perindoprilat. Peakplasma concentrations of perindoprilat are achieved 3to 4
hours after an oral dose of perindopril. Perindoprilat is about 10 to 20% bound
to plasma proteins. Perindopril is excreted predominantly in the urine,
asunchanged drug, as perindoprilat, and as other metabolites. The elimination
ofperindoprilat is biphasic witha distribution half-life of about 5 hours and
an elimination half-life of 25 to 30 hours or longer, the latter half-life
probably representing strong binding to angiotensin-converting enzyme. The
excretion of perindoprilat is decreased in renal impairment.
o Pharmacodynamic data
Perindopril, as well as other ACE inhibitors, inhibit ACE,
which isinvolved in the conversion of angiotensin I to angiotensin II.
Angiotensin II stimulates the synthesis andsecretion of aldosterone and raises
blood pressure via apotent direct vasoconstrictor effect.The pharmacological
actions of ACEinhibitors are thought to be primarily due to the inhibition of
the renin-angiotensin-aldosterone system, butsince they also effectively reduce
blood pressure in patients with low renin concentrations other mechanismsare
probably also involved.
o Clinical pharmacology
The accumulation of bradykininhas some therapeutic advantage
through vasodilatation, but isalso responsible for a dry cough in susceptible
individuals.
ACEIs can also precipitate renal dysfunction by decreasing
renal efferent arteriolar tone, thereby decreasing effective renal perfusion
pressure, a particular risk in renal artery stenosis. Otherside-effects include
hyperkalaemia due to reduced aldosterone secretion, agranulocytosis, skin
rashes, and taste disturbance. A rare idiosyncratic reaction to ACEIs can cause
angioedema with potential upper airway obstruction; this can occur several
years after initiation of ACEI therapy. ACEIs are contraindicatedin pregnancy
as they are associated with birth defects. ACEIs may interactwith drugs used
perioperatively. For example, non-steroidal anti-inflammatory drugs can
precipitaterenal dysfunction in combination with ACEIs. They can also reduce
the efficacy of ACEIs by decreasing prostaglandin synthesis.Interactions with
diuretics may cause hypovolaemia and hyponatraemia, while concurrent use of
potassium supplements orpotassium-sparing diuretics may result in
hyperkalaemia.Drugs which are renally excreted (e.g. digoxin and lithium) may
accumulatein patients taking ACEIs.
2. ARA drugs[3,71,74]
ARAs are commonly used in patients who are intolerant to
ACEIsas they are less likely to cause a dry cough.
v Losartan
o Chemical structure
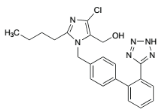 C22H22ClKN6O C22H22ClKN6O
o Pharmaceutical form and administration
Losartan is given orally as the potassium salt in tablet and
sachet forms.In hypertension the usual dose of losartan potassiumis 50 mg once
daily. The dose may be increased, if necessary, to 100 mg daily as a single
dose or in two divided doses. An initial dose of 25 mg once daily should
begiven to patients with intravascular fluid depletion, and is recommended in
the UK in patients over 75 years ofage. Similar reductions may be appropriate
in patients with hepatic or renal impairment
o Pharmacokinetic data
Losartan is readily absorbed from the gastrointestinal tract
after oral doses, but undergoes substantial firstpass metabolism resulting in a
systemic bioavailability of about 33%. It is metabolized to an active
carboxylic acid metabolite E-3174 (EXP-3174), which has greater pharmacological
activity than losartan; some inactive metabolites are also formed. Metabolism
is primarilyby cytochrome P450 isoenzymes CYP2C9 and CYP3A4. Peak plasma
concentrations of losartan andE-3174 occur about 1 hour and 3 to 4 hours,
respectively, after an oral dose. Both losartan and E-3174 aremore than 98%
bound to plasma proteins. Losartan isexcreted in the urine, and in the faeces
via bile, asunchanged drug and metabolites. About 4% of an oraldose is excreted
unchanged in urine and about 6% is excreted in urine as the active metabolite.
The terminal elimination half-lives of losartan and E-3174 are about1.5 to 2.5
hours and 3 to 9 hours, respectively.
o Pharmacodynamic data
Losartan is a competitive angiotensin II receptor antagonist
with antihypertensive activity due mainly to selectiveblockade of AT1 receptors
and the consequent reducedpressor effect of angiotensin II.
o Clinical pharmacology
The therapeutic andside-effects are broadly similar to those
of ACEIs, with evidenceof reduced risk of new onset diabetes, stroke,
progression of cardiac failure, and all-cause mortality in patients with
chronic kidney disease.Direct targeting of angiotensin II receptors has
theoretical advantages over ACE inhibition. Angiotensin II may be
producedthrough non-ACE pathways, for example, by the enzyme chymase in kidney
tissue, which is not affected by ACEIs. ARAs do not inhibit bradykinin
metabolism, and therefore, the incidence of cough is much less than with ACEIs.
The risk of angiooedema is greatly reduced with ARAs compared with ACEIs.
Losartan is contra-indicated in pregnancy.It should be used with caution in
patients with renal artery stenosis. Losartan is excreted in urine and in
bileand reduced doses may therefore be required in patients with renal
impairment and should be consideredin patients with hepatic impairment.
Patients with volume depletion (for example those who have receivedhigh-dose
diuretic therapy) may experience hypotension; volume depletion should be
corrected beforestarting therapy, or a low initial dose should be used.Since
hyperkalaemia may occur, serum-potassium concentrations should be monitored,
especially in theelderly and patients with renal impairment, and
potassium-sparing diuretics should generally be avoided.
3. Direct renin inhibitors (DRIs)[3,68]
v Aliskiren
Aliskiren, a piperidine derivative, is the only available drug
in this
class and is used by specialists in patients who are unresponsive
to, or intolerant of, other antihypertensives.
o Chemical structure
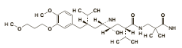 (C30H53N3O6)2,C4H4O4 (C30H53N3O6)2,C4H4O4
o Pharmaceutical form and administration
Aliskiren is given as the fumarate. Doses are expressed in
terms of the base; 165.8 mg of aliskiren fumarate is equivalent to about150 mg
of aliskiren. The usual initial oral dose of aliskiren is 150 mg once daily,
increased to 300 mg oncedaily if necessary. Doses may be taken before or
afterfood, but patients should establish a routine patternwith regard to
meals.
o Pharmacokinetic data
Aliskiren is poorly absorbed from the gastrointestinal tract
with a bioavailability of about 2.5%. Peak plasma concentrations are reached
about 1 to 3 hours after anoral dose. Absorption is reduced when aliskiren is
taken with a high-fat meal. Aliskiren is about 50% boundto plasma proteins. It
is excreted mainly in the faeces,possibly via the bile; about 25% of the
absorbed doseis excreted in the urine as unchanged drug. Aliskiren is a
substrate for the cytochrome P450 isoenzymeCYP3A4 but metabolism appears to be
minimal. The elimination half-life is about 24 to 40 hours, and steady-state
concentrations are reached in about 7 to 8days.
o Pharmacodynamic data
Aliskiren directly inhibits the enzyme renin, which is
secreted by granular cells of the juxtaglomerular apparatus. Renin inhibition
reduces theconversion of the hepatically secreted polypeptide angiotensinogen
to angiotensin I.Its effect on the RAS is therefore `upstream' of ACEIs and
ARAs and it does not cause bradykinin accumulation.
o Clinical pharmacology
DRIs have the same potential to cause renal dysfunction and
electrolyte disturbances as ACEIs and ARAs. It should therefore be used with
caution in patients with renal impairment or renovascular hypertension.
Patients withsodium or volume depletion (for example those receiving high-dose
diuretics) may experience symptomatic hypotension on starting aliskiren and
treatment shouldbegin under close medical supervision.Diarrhea is aspecific
side-effect to higher doses of DRIs. Aliskiren may have a larger role in the
management ofhypertension in future, possibly in combination with other drugs.
Aliskiren should be avoided in pregnancy since drugsacting on the
renin-angiotensin system have been associated with fetal and neonatal morbidity
and mortality.
Use of aliskiren with other antihypertensives or drugsthat
cause hypotension may have an additive effect.Renal function and electrolytes
should be monitored indiabetic patients taking aliskiren and ACE
inhibitorssince there is an increased risk of hyperkalaemia andrenal
impairment.Aliskiren is metabolised to a small extent by the cytochrome P450
isoenzyme CYP3A4 but few significant interactions have been reported.
Plasma-aliskiren concentrations may be reduced by irbesartan and increasedby
atorvastatin and ketoconazole but the clinical relevance is not clear.
Aliskiren has caused significant decreases in furosemide concentrations.
4. Adrenoceptor antagonists
a) â-Blockers[3,68,71,74]
â-Blockers are not used as first-line antihypertensives
unless there are otherindications, for example, after myocardial infarction, or
in tachyarrhythmias such as atrial fibrillation. Theirdiverse indications
include stable heart failure, thyrotoxicosis, oesophageal varices, anxiety, and
glaucoma.
â-Blockers antagonize catecholamines at
â-adrenoceptors.
These Gs type G-protein-coupled receptors are
classified as â1, present mainly within the heart and kidneys; and
â2, present throughout the body in lungs, blood vessels, and muscle. The
reduction in arterial pressure achieved by â-blockers is
attributable
to their effects upon multiple pathways. Block of â1
receptors in
the sinoatrial node reduces heart rate and block of myocardial
receptors reduces contractility (reduced chronotropy and inotropy,
respectively). They also reduce sympathetic nervous system activity, while
block of receptors in the juxtaglomerular apparatus reduces renin secretion.
v Propranolol
o Chemical structure
 C16H21NO2,HCl C16H21NO2,HCl
o Pharmaceutical form and administration
Propranolol hydrochloride is usually given orally.
Inhypertension it is given in initial doses of 40 to 80 mgtwice daily increased
as required to a usual range of 160 to 320 mg daily; some patients may require
up to640 mg daily.
o Pharmacokinetic data
Propranolol is almost completely absorbed from
thegastrointestinal tract, but is subject to considerablehepatic-tissue binding
and first-pass metabolism. Peakplasma concentrations occur about 1 to 2 hours
after anoral dose. Plasma concentrations vary greatly between individuals.
Propranolol has high lipid solubility. Itcrosses the blood-brain barrier and
the placenta, and is distributed into breast milk. Propranolol is about 90%
bound to plasma proteins. It is metabolised in the liverand at least one of its
metabolites (4-hydroxypropranolol) is considered to be active, but the
contribution of metabolites to its overall activity is uncertain.The
metabolites and small amounts of unchanged drug are excreted in the urine. The
plasma half-life of propranolol is about 3 to 6 hours.
o Pharmacodynamic data
Propranolol is a non-cardioselective â-blocker.
Propranolol also blocks sodium channels andhave membrane stabilizing activity
and is thus classed as Vaughan-Williams class 2 antiarrhythmic with other
â-blockers. In addition to their antihypertensive effect, propranolol
improves the myocardial oxygen supply:demand ratio and help reduce myocardial
ischemiaby prolonging the period of diastole.
o Clinical pharmacology
While â-blockers are used instable heart failure, they
have the potential to worsen symptomsin some patients by reducing cardiac
output. Poor peripheralcirculation and Raynaud's phenomenon may be
precipitatedboth by reduced cardiac output and block of peripheral â2
receptors. Bronchospasm caused by â2 block may be a
significantrespiratory side-effect in susceptible individuals, for
example,asthmatics. Central nervous system effects include malaise,tiredness,
and vivid dreams, particularly with lipid-solubledrugs. Ininsulin-dependent
diabetic patients, the symptoms ofhypoglycaemia may be suppressed by
sympathetic block.
b) á-Blockers[3,68,71]
á-Blockers are used to treat hypertension in patients
resistantto, or intolerant of, other treatments. Specific indications fortheir
use in secondary hypertension include labetalol for preeclampsia and
phentolamine in the perioperative management of phaeochromocytoma.
á-blockers are also commonly used to improve urinary flow in benign
prostatic hyperplasia, for
example, tamsulosin.
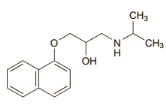
v Prazosin
o Chemical structure
 C19H21N 5O 4,HCl C19H21N 5O 4,HCl
o Pharmaceutical form and administration
Prazosin is given orally as the hydrochloride, but dosesare
usually expressed in terms of the base. Prazosin hydrochloride 1.1 mg is
equivalent to about 1 mg of prazosin.In hypertension, the usual initial dose
is500 micrograms two or three times daily for 3 to 7days; if tolerated the dose
may then be increased to1 mg two or three times daily for a further 3 to 7
days,and thereafter gradually increased, according to the patient's response,
to a usual maximum of 20 mg daily individed doses.
o Pharmacokinetic data
Prazosin is readily absorbed from the gastrointestinaltract
with peak plasma concentrations occurring 1 to 3hours after an oral dose. The
bioavailability is variableand a range of 43 to 85% has been reported.
Prazosinis highly bound to plasma proteins. It is extensivelymetabolised in the
liver and some of the metabolites are reported to have hypotensive activity. It
is excretedas the metabolites and 5 to 11% as unchanged prazosinmainly in the
faeces via the bile. Less than 10% is excreted in the urine. Small amounts are
distributed intobreast milk. Its duration of action is longer than wouldbe
predicted from its relatively short plasma half-life ofabout 2 to 4 hours.
Half-life is reported to be increasedto about 7 hours in patients with heart
failure.
o Pharmacodynamic data
Prazosin is an alpha blocker that acts by selective blockade
of alpha1-adrenoceptors.Prazosin produces peripheral dilatation of both
arterioles and veins and reduction of peripheral resistance,usually without
reflex tachycardia. It reduces bothstanding and supine blood pressure with a
greater effect on the diastolic pressure.
o Clinical pharmacology
Treatment with prazosin should be introduced cautiously
because of the risk of sudden collapse following the initial dose. Extra
caution is necessary in patients with hepatic or renal impairment and in
theelderly.Prazosin is not recommended for the treatment of heart failure
caused by mechanical obstruction, for exampleaortic or mitral valve stenosis,
pulmonary embolism,and restrictive pericardial disease. It should be used with
caution in patients with angina pectoris. Prazosinmay cause drowsiness or
dizziness; patients so affected should not drive or operate machinery.The
hypotensive effects of prazosin may be enhancedby use with diuretics and other
antihypertensives, andby alcohol and other drugs that cause hypotension.
Therisk of first-dose hypotension may be particularly increased in patients
receiving beta blockers or calcium-channel blockers.
5. Calcium channel blockers
(CCBs)[3,68,71,74]
CCBs are first-line treatment for primary hypertension in
patients over the age of 55 and black patients of African orCaribbean family
origin. Rate-controlling CCBs (diltiazem, verapamil) are also used to manage
tachyarrhythmias and angina,where their negative inotropic and chronotropic
effects improvethe myocardial oxygen supply. Some CCBs havespecific non-cardiac
indications, for example, nimodipine inneurosurgery to reduce cerebralvasospasm
in patients afterspontaneous subarachnoid hemorrhage, and verapamil inneurology
to treat cluster headache.
v Amlodipine
o Chemical structure
 C20H25ClN2O5,C6H6O3S C20H25ClN2O5,C6H6O3S
o Pharmaceutical form and administration
Amlodipine is given orally as the besilate, but doses are
usually expressed in terms of the base; amlodipine besilate 6.9 mg is
equivalent to about 5 mg of amlodipine. The camsilate, maleate, and mesilate
are alsoused.In hypertension the usual initial dose is 5 mg once daily,
increased, if necessary, to 10 mg once daily.
o Pharmacokinetic data
Amlodipine is well absorbed after oral doses with peakblood
concentrations occurring after 6 to 12 hours. Thebioavailability varies but is
usually about 60 to 65%.Amlodipine is reported to be about 97.5% bound toplasma
proteins. It has a prolonged terminal elimination half-life of 35 to 50 hours
and steady-state plasmaconcentrations are not achieved until after 7 to 8
daysof use. Amlodipine is extensively metabolized in theliver; metabolites are
mostly excreted in urine togetherwith less than 10% of a dose as unchanged
drug.
o Pharmacodynamic data
Amlodipine as well as other dihydropyridines act on L-type
calcium channels present in vascularsmooth muscle and in myocardial and nodal
tissues. The variable affinity of the different CCBs to these different tissues
determines their effects.
o Clinical pharmacology
Cardiovascular side-effects include reflex tachycardia which
may potentiate myocardial ischemia, disturbance of the peripheral
microcirculation leading to swelling of the hands and feet, flushing, and
headache. Rate-limiting agents prolongatrioventricular conduction and cause
bradycardia; the negativeinotropic and chronotropic effects may worsen heart
failure. Amlodipine may enhance the antihypertensive effects ofother
antihypertensive drugs such as beta blockers although the combination is
generally well tolerated. Enhanced antihypertensive effects may also be seen
ifused with drugs such as aldesleukin and antipsychotics that cause
hypotension. Amlodipine may modify insulinand glucose responses and therefore
diabetic patientsmay need to adjust their antidiabetic treatment when receiving
amlodipine. Amlodipine is extensively metabolized in the liver by the
cytochrome P450 isoenzymeCYP3A4, and interactions may occur with other
drugs,such as quinidine, sharing the same metabolic pathway, and with enzyme
inducers, such as carbamazepine, phenytoin, and rifampicin, and
enzymeinhibitors, such as cimetidine, erythromycin, and HIVprotease
inhibitors.
6. Diuretics[3,68,71,72,74]
Thiazide (bendroflumethiazide, hydrochlorothiazide) and
thiazide-like (chlortalidone, indapamide) diuretics are the most commonly
prescribed diuretic agents used to treat hypertension.They are used in patients
intolerant of CCBs and in patients with heart failure, or at risk of heart
failure. They are also usedas `add on' drugs in patients who have not responded
to firstand second-line antihypertensive treatments.
o Chemical structure
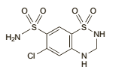 C7H8ClN3O4S2 C7H8ClN3O4S2
o Pharmaceutical form and administration
Thiazides are usuallygiven in the morning so that sleep is not
interrupted by diuresis.Hydrochlorothiazide is given orally.In the treatment of
hypertension an initial dose of12.5 mg may be sufficient, increasing to 25 to
50 mgdaily if necessary, either alone or with other antihypertensives. Doses of
up to 100 mg have been suggestedbut are rarely necessary.
o Pharmacokinetic data
Hydrochlorothiazide is fairly rapidly absorbed fromthe
gastrointestinal tract. It is reported to have a bioavailability of about 65 to
70%. It has been estimated tohave a plasma half-life of between about 5 and
15hours and appears to be preferentially bound to redblood cells. It is
excreted mainly unchanged in theurine. Hydrochlorothiazide crosses the
placental barrier and is distributed into breast milk.
o Pharmacodynamic data
Thiazides are moderately potent diuretics and exerttheir
diuretic effect by reducing the reabsorption ofelectrolytes from the renal
tubules, thereby increasing the excretion of sodium and chloride ions, and
consequently of water. They act mainly at the beginning ofthe distal tubules.
The excretion of other electrolytes,notably potassium and magnesium, is also
increased.The excretion of calcium is reduced. They also reduce
carbonic-anhydrase activity so that bicarbonate excretion is increased, but
this effect is generally small compared with the effect on chloride excretion
and does notappreciably alter the pH of the urine. They may also reduce the
glomerular filtration rate.Their hypotensive effect is probably partly due to a
reduction in peripheral resistance; they also enhance theeffects of other
antihypertensives. Paradoxically, thiazides have an antidiuretic effect in
patients with diabetes insipidus.
o Clinical pharmacology
Thiazide diuretics have many clinically relevant
biochemicalside-effects including hypokalaemia, hypercalcaemia, hyponatraemia,
hypomagnesaemia, hyperglycaemia, hyperuricaemia, hypercholesterolaemia, and
hypochloraemic alkalosis. Plasma volume loss may precipitate dehydration and
acute kidney injury. Less common side-effects include skin rashes,
photosensitivity reactions, and blood dyscrasias including
thrombocytopaenia.
Many of the interactions of hydrochlorothiazide andother
thiazides are due to their effects on fluid and electrolyte balance.
Diuretic-induced hypokalaemia mayenhance the toxicity of digitalis glycosides
and mayalso increase the risk of arrhythmias with drugs that prolong the QT
interval, such as astemizole, terfenadine, halofantrine, pimozide, and sotalol.
Thiazidesmay enhance the neuromuscular blocking action of competitive
neuromuscular blockers, such as atracurium, probably by their hypokalaemic
effect. The potassium-depleting effect of diuretics may be enhanced
bycorticosteroids, corticotropin, beta2 agonists such as salbutamol,
carbenoxolone, amphotericin B, or reboxetine.
Diuretics may enhance the effect of other antihypertensives,
particularly the first-dose hypotension that occurs with alpha blockers or ACE
inhibitors. Orthostatichypotension associated with diuretics may be enhanced by
alcohol, barbiturates, or opioids. The antihypertensive effects of diuretics
may be antagonised bydrugs that cause fluid retention, such as
corticosteroids,NSAIDs, or carbenoxolone; diuretics may enhance the
nephrotoxicity of NSAIDs. Thiazides have been reported to diminish the response
to pressor amines, suchas noradrenaline, but the clinical significance of this
effect is uncertain.Thiazides should not usually be used with lithium sincethe
association may lead to toxic blood concentrations of lithium. Other drugs for
which increased toxicity hasbeen reported when given with thiazides include
allopurinol and tetracyclines. Thiazides may alter the requirements for
hypoglycaemics in diabetic patients.
Other diuretics include aldosterone antagonists, for example,
spironolactone, are recommended as fourth-line treatment of primary
hypertension. The use of these drugs carries a risk of hyperkalaemia,
particularly in patients with impaired renal function or who are taking other
potassium-sparing agents. Loop diuretics are indicated for resistant
hypertension in patients with heart failure, chronic kidney disease, and in
those at risk of hyperkalaemia.
· Other antihypertensives
1. Vasodilators[3,68,71]
Directly acting vasodilators, for example, hydralazine
andminoxidil, are seldom used due to their side-effect profiles.Hydralazine is
used in hypertension secondary to pre-eclampsia. In addition to its
antihypertensive effects, minoxidil is used topically as a treatment for male
pattern baldness.
v Minoxidil
o Chemical structure
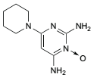 C9H15N5O C9H15N5O
o Pharmaceutical form and administration
In the treatment of hypertension minoxidil is givenwith a beta
blocker, or with methyldopa, to diminishthe cardiac-accelerating effects, and
with a diuretic,usually a loop diuretic, to control oedema.An initial dose of 5
mg of minoxidil daily(or 2.5 mg daily in the elderly) is gradually increased
atintervals of not less than 3 days to 40 or 50 mg dailyaccording to response;
in exceptional circumstances up to 100 mg daily has been given.
In the treatment ofalopecia androgenetica (male-pattern
baldness) 1 ml of a 2% or 5% solution of minoxidil is applied twice daily to
the scalp. The 5% solutionis not recommended for women.
o Pharmacokinetic data
About 90% of an oral dose of minoxidil is absorbedfrom the
gastrointestinal tract. The plasma half-life isabout 4.2 hours although the
haemodynamic effectmay persist for up to 75 hours, presumably due to
accumulation at its site ofaction. Minoxidil is not boundto plasma proteins. It
is distributed into breast milk.Minoxidil is extensively metabolised by the
liver. It requires sulfation to become active, but the major metabolite is a
glucuronide conjugate. Minoxidil is excretedpredominantly in the urine mainly
in the form ofmetabolites. Minoxidil and its metabolites are dialysable,
although the pharmacological effect is not reversed. About 0.3 to 4.5% of a
topical dose of minoxidil is absorbed from intact scalp.
o Pharmacodynamic data
Vasodilators cause relaxation of vascular smooth muscle in
resistance (arteriolar) vessels. Minoxidil achieves this via adenosine
triphosphate-dependent potassium channels on smoothmuscle cell
membranes.Vasodilatation provokes reflex cardiac stimulation (which may
precipitate cardiac ischemia) and RAS activation. These compensatory responses
may be offset by â-blockersor diuretics.
o Clinical pharmacology
Vasodilator drugs are poorly tolerated. Side-effects
includeheadache, fluid retention, and edema. Other specific side effects
include left ventricular hypertrophy, pericardial and
pleural effusions,
hypertrichosis and coarsening of featureswith minoxidil, while peripheral
neuropathy, blood dyscrasias,and a lupus-like reaction can occur with
hydralazine.The antihypertensive effect of minoxidil may be enhanced by use of
other hypotensive drugs. Severeorthostatic hypotension may occur if minoxidil
andsympathetic blocking drugs such as guanethidine aregiven
concurrently.Topical minoxidil should not be used with other topicalagents
known to enhance absorption, such as corticosteroids, retinoids, or occlusive
ointment bases.
2. Centrally acting agents[3,68,71,74]
Centrally acting agents include clonidine (á2
adrenoceptor agonist), methyldopa (precursor of an á2 adrenoceptor
agonist), and moxonidine (agonist at imidazoline binding sites). Their use
inprimary hypertension is limited to difficult to treat cases, while methyldopa
is used to treat hypertension in pregnancy. The evidence base for the use of
centrally acting drugs in hypertension is limited and adverse effects are
common. Clonidine is an analgesic and sedative drug which reduces the
minimumalveolarconcentration of inhalation anestheticagents. Both of these
drugs cause side-effects including drymouth and sedation. Methyldopa has
immunological side effects, including pyrexia, hemolytic anemia, and
hepatitis.Cessation of treatment with clonidine can cause
reboundhypertension.
v Methyldopa
o Chemical structure
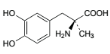 C10H13NO4,1 / H2O C10H13NO4,1 / H2O
o Pharmaceutical form and administration
Methyldopa is given orally as the sesquihydrate, butdoses are
usually expressed in terms of anhydrousmethyldopa. Methyldopa sesquihydrate
1.13 g isequivalent to about 1 g of anhydrous methyldopa. Forhypertensive
crises, methyldopa has been given intravenously as methyldopate
hydrochloride.In hypertension, the usual initial adult oral dose is250 mg of
methyldopa two or three times daily for 2days; this is then adjusted, not more
frequently than every 2 days according to response, up to a usual maximum dose
of 3 g daily. The usual maintenance dosage is 0.5 to 2 g of methyldopa daily.
In the elderly an initial dose of 125 mg twice daily has been used; this
dosemay be increased gradually if necessary, but should not exceed 2 g daily
o Pharmacokinetic data
After oral use methyldopa is variably and
incompletelyabsorbed, apparently by an amino-acid active transportsystem. The
mean bioavailability has been reported to be about 50%. It is extensively
metabolised and is excreted in urine mainly as unchanged drug and the Osulfate
conjugate. It crosses the blood-brain barrier andis decarboxylated in the CNS
to active alpha-methylnoradrenaline.The elimination is biphasic with a
half-life of about 1.7 hours in the initial phase; the second phase is more
prolonged. Clearance is decreased and half-life prolongedin renal impairment.
Plasma protein binding is reported to be minimal. Methyldopa crosses the
placenta; smallamounts are distributed into breast milk.
o Pharmacodynamic data
Methyldopa is an antihypertensive that is thought tohave a
mainly central action. It is decarboxylated in theCNS to
alpha-methylnoradrenaline, which is thoughtto stimulate alpha2 adrenoceptors
resulting in a reduction in sympathetic tone and a fall in blood pressure.
Itmay also act as a false neurotransmitter, and have some inhibitory actions on
plasma renin activity. Methyldopa reduces the tissue concentrations of
dopamine,noradrenaline, adrenaline, and serotonin.
o Clinical pharmacology
Methyldopa should be used with caution in the elderly,and in
patients with hepatic or renal impairment or witha history of haemolytic
anaemia, liver disease, or depression. Care is also advisable in patients with
Parkinsonism. It should not be given to patients with active liver disease or
depression and it is not recommendedfor phaeochromocytoma.It is advisable to
make periodic blood counts and toperform liver function tests at intervals
during the first6 to 12 weeks of treatment or if the patient develops
anunexplained fever. Patients taking methyldopa mayproduce a positive response
to a direct Coombs' test; if blood transfusion is required, prior knowledge of
apositive direct Coombs' test reaction will aid crossmatching. Methyldopa may
cause sedation; if affected, patients should not drive or operate machinery.
The hypotensive effects ofmethyldopa are potentiatedby diuretics, other
antihypertensives, and drugs withhypotensive effects. However, there have been
reportsof paradoxical antagonism of the hypotensive effectsby tricyclic
antidepressants, antipsychotics, and betablockers. Sympathomimetics may also
antagonise the hypotensive effects.There may be an interaction between
methyldopa andMAOIs and care is required if they are given together.Caution is
also needed with catechol-O-methyltransferase inhibitors, such as entacapone,
since they might reduce the metabolism of methyldopa.
3. Ganglion blockers[3,68]
Ganglion blockers, such as trimetaphan, antagonize
acetylcholine at nicotinic receptors, including those at the adrenal cortex.
Trimetaphan causes vasodilatation with a consequent rapid reduction in arterial
pressure. Although ganglion blockers may be
used to manage hypertensive
crises or to provide hypotensive anesthesia, their use is increasingly rare.
The drug regimenmay include drugs with differing
pharmacological actions; the antihypertensive mechanism is not fully understood
in all cases. Historically, thiazide diuretics and beta blockers have been the
mainstay of drug therapy for hypertension, but CCBs, ACEI, ARAs, and alpha
blockers are now also widely used[3].
Studies such as the TOMHS[75] (comparing
chlortalidone, acebutolol, amlodipine, enalapril, and doxazosin), and a similar
study[76] (comparing hydrochlorothiazide, atenolol, diltiazem,
captopril, prazosin, and clonidine), have shown that the main types of
antihypertensive drug reduce blood pressure to a similar extent and in a
similar proportion of patients, although the response may also depend on
individual factors such as age[77] and
race[78,79]. ARAs also effectively reduceblood pressure.
However, it is now generally acknowledgedthat a single drug is unlikely to
control blood pressureadequately and most patients will require more thanone
drug to reach their treatment target. Tolerance of thedrug groups is also
similar, although there has been concernabout the metabolic effects of
thiazides and betablockers. Alpha blockers (specifically
doxazosin[80]) havebeen associated with an increased risk of
heart failure,which may limit their use. The safety of short-acting
dihydropyridine CCBs has also been questioned,and they are no longer generally
recommended forhypertension[3]; long-acting
dihydropyridines,however, are of established benefit[81].
Diuretics(particularly thiazides) and beta blockers were the firstdrugs to
demonstrate an effect on mortality in long-termoutcome studies and have
therefore been preferred for initialtherapy[3]. However,
long-term studies with other druggroups have now been performed, and have
generallyshown comparable effects on mortality and morbidity. Ameta-analysis
concluded that there was little differencein overall cardiovascular outcomes
for regimens based on ACEIs, ARAs, CCBs, beta blockers, or diuretics,
suggestingthe major benefit of treatment related to reduction of blood pressure
rather than to specific properties of the individualdrugs[82].
In general, guidelines acknowledge that lowering bloodpressure appears to be
more important than which drug ischosen for initial therapy, and that most
patients will requirea combination of drugs, making the initial choice
lessimportant[3]. Thiazide diuretics, ACEIs, ARAs, or CCBs
mayall be used, and choice should take into account individualpatient
characteristics, including age, ethnicity, contra-indicationsor compelling
indications for specific drugs, adverseeffects, and relative
cost-effectiveness[40,59-61]. Strictguidance is therefore not
generally given, although foruncomplicated patients WHO
guidelines[59]recommend thiazide diureticsas first-line,
whereas in theUK[81] diuretics or CCBsare recommendedfor older
patients (55 years or over) and black patients,while in younger, non-black
patients ACEIs or ARAsare preferred. Compellingindications in all the
guidelines include the use of ACEIs or ARAs in patientswith nephropathy,
diuretics or CCBs in elderly patients, and beta blockers in patientswho have
had a myocardial infarction.
Having decided what drug to use, treatment is started at
thelowest recommended dose. If this is ineffective or onlypartially effective
the dose may be increased (except in thecase of thiazide diuretics where there
is generally no additionalbenefit, but more adverse effects);
alternativelyanother first-line drug may either be substituted (sequential
therapy) or added (combination therapy). Two-drugcombinations will control
blood pressure in a higher proportionof patients and may be necessary in most
patientsto achieve optimal levels, although the effects of the twodrugs may not
be fully additive. Combination therapy alsoallows lower doses of the individual
drugs to be used witha consequent reduction in adverse effects. Initial
treatmentwith a low-dose combination may be considered in
somepatients[40,61].
The most effective combinations involve drugsthat act on
different physiological systems. Appropriatecombinations therefore
include[3]:
Ø diuretic plus beta blocker
Ø diuretic plus ACEI
Ø diuretic plus ARA
Ø CCB plus ACEI
Ø CCB plus ARA
Ø CCB (except verapamil) plus betablocker
Alpha blockersmay be used with any of the other classesbut are
usually reserved for third-line therapy unless specificallyindicated for
another reason. A 3-drug combinationis often required, especially in severe
hypertension. Inpatients who maintain an elevated diastolic blood
pressuredespite triple therapy the possibility of secondary hypertensionshould
be considered, although factors such as non-compliance, NSAID use, or alcohol
abuse may contributeto resistance[83,84].
Other classesof antihypertensive drugs that are sometimesused
include: centrally acting drugs such as clonidine,methyldopa, and the less
sedating moxonidine; direct-actingvasodilators such as hydralazine and
minoxidil; the aldosteroneantagonist, eplerenone; and the
DRI,aliskiren[3]. Endopeptidase inhibitors and
endothelinantagonists are among various drug groups thatare under
investigation.
It has been standard teachingthat drug treatment for
hypertension is continued indefinitely,but there have been some reports of
successfulwithdrawal in selected patients[85,86]. If this is
attempted,blood pressure must be closely monitored and lifestylemeasures should
be continued indefinitely[40,81].
G. ADHERENCE TO ANTIHYPERTENSIVE THERAPY
The mainobjective of hypertension management is to achieve BP
control through changes in lifestyle andappropriate therapeutic
measures[1,3]. In the recent years,a growing number of
patients seem to present resistanceto the antihypertensive
treatment[87]. Non-adherence has important economic
implications: itdecreases the cost-effectiveness of interventions, leading
topoor clinical results with increased costs for public
health[88].
Different methods to measure therapeutic adherence
areavailable; all have favourable and unfavourable aspects andthere currently
is no gold standard[10]. These methods canbe classified as
direct or indirect: interviews and questionnairesadministered to patients (one
of the most used isMorisky's scale), diaries, pill count, electronic
monitoringof orally administered drugs, rates of prescriptions'
refillingthrough pharmacies' databases and direct dosage ofdrug levels in
biological fluids[10].At the present time therapeutic drug
monitoring (TDM)is available for most antihypertensive drugs and severalstudies
have demonstrated its efficacy in the evaluation
ofadherence[89,90].
1. Definition and Forms of Non Adherence
Therapeutic adherence describes the extent to which patients
actively take medications as prescribed by their health care providers based on
a therapeutic alliance or contract established between them; it differs from
the term compliance, withwhich it is often confused[10]. The
term compliance suggests apassive attitude when the patient follows the
physician'sinstructions and treatment plan, without a real
therapeuticalliance[10].In any case, these two terms do not
allow to differentiatepatients who systematically fail to follow the
recommendationsfrom those who occasionally forget a pill or reducethe dose of
the drug[91].
Several conditions could affect therapeutic adherence:type of
prescribed therapy, patient socio-cultural level,frequency of clinical
controls[92]. The WHO hasidentified five different
conditions[23] correlated with pooradherence: social and
economic factors, condition-relatedfactors, therapy-related factors,
patient-related factors andhealth care system/health care team-related factors.
Moreover,frequently patients start the prescribed therapy, butadherence is
progressively reduced because they do notpersist[23].
2. Methods for assessing drug adherence
Different methods to measure therapeutic adherence
areavailable. Indirect methods are less invasive andless expensive. Indirect
methods for evaluating drug adherence include: patient interview, diaries,
questionnaires,pill count, review of prescriptions, electronic
monitoring[15,21].Conversely, direct methods are more
invasive, expensiveand include the direct observation of the patient duringthe
administration of therapy and the measurement of thedrug or of its metabolites
in blood or urine[93]. Nonetheless, only a limited number of
drugs can be monitored in this way because the bioavailability and
completenessof absorption of various drugs, as well as the rate of
metabolismand excretion, are factors that make it difficult tocorrelate drug
concentrations in blood or urine with adherence[15].
In clinical practice, each method has advantages
anddisadvantages and not all can be easily used in all settings;it is often not
possible to accurately determine the realdegree of adherence.
2.1 Patient Interview[15,21,94]
The patient interview is one of the simplest and lessexpensive
methods, but its efficacy is strongly influenced bythe ability of the
interviewer and by the way in which thequestions are asked; it also heavily
relies on a strongdoctor-patient relationship. Interviews may allow a
pharmacist to show concern for the patient andprovide immediate feedback.
Typical questions include askingthe patient to report the name, the dosage and
the timeof drug intake. Alternatively, the doctor may ask the patienthow often
they forget to take the pills during a week or amonth; according to the answers
the physician can theninfer the degree of adherence.The patient interview can
also be targeted to encouragebehavioural changes and to improve therapeutic
adherence.
A drawback of this method isthat it can overestimate
adherence, and its accuracy dependson the patient's cognitive abilities and the
honesty of replies,as well as the interviewer's correct interpretation of
responses.
2.2 Questionnaires[94,95]
The questionnaires are simple and economical methods
toevaluate adherence. They are usually composed of a limitednumber of questions
that the patient is asked to answer.A potential disadvantage is represented by
the difficultysome subjects may have understanding the questions incase of low
level of education.One of the most frequently used questionnaire is theMorisky
8 items MMAS-8 (Eight-Item Morisky MedicationAdherence Scale), which has a
higher specificity(93%) compared to the original 4-item questionnaire.The
MMAS-8 consists of seven binary questions (YES/NO) and one last multiple choice
question. A clearlimitation of this instrument is represented by patients
whomight answer the questions untruthfully. Similarly to thepatient interview,
questionnaires are useful to evaluateunintentional poor adherence.
2.3 Pill Count
Counting pills is a simple and cost-effectiveness method
toevaluate therapeutic adherence, but it is characterized byseveral
limitations[94]. Among these, the most relevantlimitation is
represented by the fact that patients canmislead the doctor, throwing away the
pills before the visit.Furthermore, this method does not provide
accurateinformation on daily adherence[10].The accuracy of the
pill count method is adequate when compliance is excellent because there is
nothing to return; however, in cases of low compliance, the pill count is not
accurate.
An effective but at the same time more complex andexpensive
method is electronic monitoring, in which thepill count is performed
automatically and continuously by adevice at the patients'
home[96].
2.4 Electronic Monitoring[94,97]
There are different electronic monitoring systems, usuallyvery
expensive and invasive. However, in view of theirhigh efficacy they can be
considered as the gold standardfor assessing drug adherence.One of the less
invasive options is the medication events monitoring system (MEMS), which uses
a device to recordthe time and the date in which medications are taken fromthe
box. In the last years, a more invasive system was validated: the ingestible
sensor technology. In thiscase the sensor is inside the pill, so it becomes
possible toaccurately document each time a pill is ingested.
2.5 Therapeutic Drug Monitoring (TDM)
The most accurate direct method for evaluating
therapeuticadherence is the measurement of the drug concentration (orthe
concentration of its metabolites) in body fluids (bloodor
urine)[94]. For this purpose, liquid
chromatography-massspectrometry analysis can be used[93].In
the recent years, several studies used TDM to evaluatetherapeutic adherence,
especially in patients withsuspected resistant
hypertension[89,90]. Chung et al.[98]have
recently demonstrated that TDM is a cost-effectivemethod to assess adherence in
the workup of resistanthypertension, independently by sex and age. TDM
wouldallow a substantial economic advantage when comparedwith previous
cost-effectiveness analyses of invasive proceduresfor the treatment of
resistant hypertension[99,100].
TDM, however, has some limitations: it provides resultswithout
giving information about the causes of non-adherence;it is very intrusive for
the patient; it can induce thephenomenon of white-coat adherence;
antihypertensivedrugs can present an altered metabolism when taken withother
drugs. An ethical problem should also be considered:such measurements need to
be done with the informedconsent of patients and thus tend to induce
white-coatadherence[94]. Despite these limitations, TDM, used
inselected cases, may provide a way to decrease health costs, by reducing the
number of visits in patients who intentionallydo not take drugs and by
identifying those patientswho would undergo unnecessary and sometimes
invasiveprocedures.
3. Improving Therapeutic Adherence[21,101]
Properly identify patients who do not take prescribedtherapy
has important consequences, such as execution ofunnecessary medical visits or
diagnostic tests, as well asscreening evaluation for secondary forms of
hypertension.For this reason it is important to improve therapeuticadherence in
all patients with hypertension, mostly in thosewith difficult to treat or
resistant hypertension. Although itis a difficult task, physician should help
individual patientto get better therapeutic compliance.
There are different strategies for improving long
termtherapeutic adherence: simplifying medical regime withfixed associations of
drugs when available, giving patientsinstructional material, counselling about
therapy, remindingfor medications and appointments, cuing medications todaily
events and explicitly acknowledging patient's effortto adhere.Therefore,
treating physician should establish a goodrelationship with each individual
patient to address andresolve poor therapeutic adherence.
4. Reasons for poor therapeutic adherence
v Non-African countries
In a cross-sectional study carried out by Lin et al. in 1995
in Tainan City, the medication adherence rate found was 57.6%. Subjects taking
80% or more of their prescribed medicines were considered
adherent[102]. The factors associated significantly with poor
adherence included: more than once daily dose frequency, no health check-up in
the previous year, disbelief in the efficacy of antihypertensives, and presence
of adverse drug reactions in the past 6 months.
In a cross-sectional study by Hashmi et al. in 2005 in
Pakistan, 77% of their cases were adherent. Univariate analyses showed that
decreasing age, poor awareness and decreasing number of pills prescribed
significantly promoted poor adherence[103].
In Bangladesh, Hussain et al. found in 2006 that 85% of the
study population were non-adherent to treatment. The reasons pointed out were
low levels of education, low family income, duration of diagnosis, insufficient
knowledge of the disease, lack of accompanying person to go to the
physician/hospital, and deficiencies in information from the service
provider[104].
In a cohort study carried out in Italy involving 18,806 newly
diagnosed hypertensive patients =35 years of age, Mazzaglia et al. in 2009
found varying adherence rates. 6 months after index diagnosis, the authors
found rates of 8.1%, 40.5%, and 51.4% corresponding to high, intermediate, and
low adherence levels, respectively. The authors also found thatthe risk of
being a poor adherer increased in the absence of concurrent
treatment[105].
In a descriptive exploratory study conducted by Demoner et al.
in 2011 in Brazil, 64% of patients in the study were nonadherent to
antihypertensive therapy. Adherence level was assessed using the Morisky-Green
Test. Poor adherence was significantly associated with participants who were:
in the youngest age group, working, lack of understanding of the health team
recommendations and presenting with overweight or
obesity[106].The younger patients (18-40 years) presented
lowerlevels of adherence compared to those in older agegroups. This may be
related to the fact that HTN is asilent disease and, thus, leads to a certain
nonchalance inyounger individuals regarding the control of the disease,who only
give importance to adequate treatment whenthere is a worsening of symptoms,
increasing risks ofserious complications and mortality for stroke and MI.
In a cross-sectional study carried out in 2014 in Iran,
Behnood-Rod et al. found that about half of the sample population (49.6%)
showed low adherence (8-item Morisky Medication Adherence Scale <6). In
addition the authors also found that overweight/obesity, previous history of
admission to emergency services due to hypertensive crisis, and getting
medication directly from drugstore without refill prescription in hand were
factors recognized to have statistically significant association with the
8-item Morisky Medication Adherence Scale score[107].
In a retrospective cohort study including 5025 Swedish adult
patients in 2015, Hedna et al. found that non-adherence to any antihypertensive
medication was higher among persons < 65 years (17.5%) and with the lowest
income (12.8%). Also the authors found that, non-adherence to the full AHT
regimen was higher among new users (44.2%), persons using specialized
healthcare (38.3%) and having multiple antihypertensive medications (54.3%).
Finally, the authors noted that non-adherence to any antihypertensive
medication a month prior to healthcare visit wasassociated with elevated
BP[108].
In cross-sectional correlational study by Lo et al. in Hong
Kong in 2016, more than half of the respondents (55.9%) acknowledged some
degree of medication nonadherence. Older age, living alone, and perception
related to treatment control were independently associated with increased odds
of medication non-adherence[109].
v African countries
In a cross sectional study carried out in Northwest Ethiopia
in 2011, Ambaw et al. found that more than half (64.6%) of the study
participants were found to be adherent to their treatment. Male gender,
insufficient knowledge about hypertension and its treatment, distance from the
hospital and presence of comorbidity were found to be significantly associated
with poor treatment adherence[7].
In 2013, Okwuonu et al. found in a cross-sectional study
carried out in Nigeria that BP control was optimal in 33%, good knowledge of
hypertension was found in 52% and only 31.8% were adherent to prescribed
medications. The authors also added that the duration ofhypertension from time
of diagnosis, systolic and diastolic blood pressures andtotal number of pills
swallowed per time were found to independently correlatewith medication
adherence, albeit negatively[110].
In a multicenter cross-sectional study conducted in 2013 by
Boima et al., medication non-adherence was found in 66.7% of the study
population. Medication non-adherence showed correlation with depression,
concern about medications, and knowledge of hypertension. Adherence was
associated with formal education and use of herbal preparation. Poor BP control
was observed in 69.7% and there was significant association between medication
non-adherence and poor BP control[111].
v In Cameroon
Tufon et al. found in a cross-sectional study carried out at
the Mankon Sub-Divisional Health Center in 2014 that level of adherence was
found to be low (80.0%) with smoking (85%) and alcohol intake (55%) identified
as associated factors. Moderate level of knowledge (77.5%) was found as opposed
to a high level of adherence (80.0%) and the authors found no significant
relationship between the level of knowledge and level of
adherence[18].
Essomba et al. reported in 2015 in a cross-sectional and
analytical study, that 26.2% of their study population had good adherence to
antihypertensive treatment. At the same time, 25.7% were bad adherers. Reasons
for non-adherence included: forgetfulness, medication cost and poor knowledge
of the disease[19].
Akoko et al. in 2015 reported that antihypertensive compliance
rate was 43.9% in their cross-sectional study. Independent predictors of
noncompliance were forgetfulness, lack of motivation due to the incurable
nature of the disease, and lack of symptoms of the
disease[20].
Mbouemboue et al. reported in 2016 that, 12.9% of patients
enrolled in the study in the Medico-Social Centre of the National Social
Insurance Fund in Garouafollowed up their treatment correctly, 52.9% had minor
observance problems, and 34.3% had a poor therapeutic adherence to
antihypertensive drugs. Therapeutic adherence was evaluated on the basis of the
Girerd X observance test. The determining factors of poor adherence were the
presence of complications of high BP, the presence of a handicap, and a low
level of education[16].
| 


