RESUME
Introduction ; Les infections urinaire sont
les invasions pathologique de voies urinaire par des micro-organismes. Il
constitue un problème majeur de sante publique en termes de
morbidité et cout financier. Les infections urinaires sont
considérées comme l'une des infections bactériennes les
plus courantes acquises dans la communauté et dans les hôpitaux.
Cette étude avait pour objectif d'étudier le profil des
micro-organismes impliques dans les infections urinaire et leurs profil de
sensibilité aux antimicrobiens courant chez les patients
fréquentant l'hôpital de district de Nylon.
Méthode : Pour obtenir cela, les informations sur les
patients ont été collectées à partir des registres
du laboratoire de 2019 (Décembre à Juin). Ces données ont
ensuite été analysées à l'aide du logiciel graph
part version 20.1 et le test de Chi Carre a été utilisé
pour comparer les variables. Résultats : Des 248
participants ,79.03% étaient des femmes tandis que 20.97% des hommes.
Les patients entre les tranches d'âge 21-30 et 1-10 étaient plus
répandus tandis que ceux d'entre 71-80 étaient moins
répandus .E coli était l'uropathogen le plus infectieux (31.45%),
suivi des espèces de staphylocoque (27.02%). Les infections multiples
ou mixes étaient courantes avec E coli et les espèces de candida
(2.42%). Les hommes étaient plus infecter avec les espèces de
klebsielles, proteus et staphylocoque, tandis que les femmes étaient
plus infecter avec E. coli, Providencia stuartii et Candida albicans. Une
prévalence élevée a été observée dans
les tranches d'âge [21-30] (29.84%), [1-10] (18.95%),[31-40] (16.53%) et
[11-20] (10.08%) respectivement. La répartition des micro-organismes en
fonction de leurs sensibilité aux classes d'antimicrobiens montre que
candida albicans était plus sensible à la fois dans les azoles
(75.00%) et les polyenes (58.33%). E. coli était plus sensible aux
macrolides (78.21%) tandis qu'il était résistant aux
céphalosporines (55.13%), Pénicilline (84.62%), fluoroquinolones
(68.57%) et carbapeneme (58.79%). Conclusion : Cette
étude révèle un schéma familier avec les
uropathogens impliques dans les infections urinaires, la principale cause
étant les bactéries gram négatives, E. coli et les
espèces de Klebsielle comme les principaux .Les bactéries gram
positive étaient également parmi l'étiologie, les
espèces de staphylocoque étant le principal. Les champignons
aussi étaient présents .Cette étude a également
montré une résistance bactérienne considérable aux
antimicrobiens prescrit empiriquement.
Mots clé : Infections urinaire,
Uropathogens, Profile de sensibilité.
LIST OF ACRONYMS AND ABBREVIATIONS
ATP: Adenosine Triphosphate
CFU: Colonies Forming Unit
CLED: Cysteine Lactose Electrolyte Deficient
CM: Centimeters
DHN: District Hospital of Nylon
DNA: Deoxyribonucleic Acid
EMB: Ethylene Methylene Blue
H2S: Hydrogen Sulphide
HAUTI: Hospital Acquired Urinary Tract Infections
HIV: Human Immunodeficiency Virus
HPF: High Power Field
IHC: Integrated Health Center
IMP: Infant and Maternal Protection
KIA: Kligger Ion Agar
LAP: Lower Abdominal Pains
LE: Leukocyte Esterase
ML: Millilitres
NA: Not Applicable
P.areoginosa; Pseudomonas aeroginosa
P.stuartii; Providencia stuartii
SP: Specie
UPEC: Uropathogenic Escherichia Coli
UTI; Urinary Tract Infections
WBC: White Blood Cells
WHO: World Health Organization
LIST OF TABLES
Table 1; Macroscopy of urine and its
implication
Table 2: previous study related to the topic
Table 3: Repartition of the microorganism in the
study population
Table 4: Repartition of the microorganism
according to sex
Table 5: Repartition of microorganisms according
to the age range
Table 6: Repartition of microorganism according
to their susceptibility to classes of antimicrobial drugs
Table 7; Comparison of the susceptibility of
microorganisms to classes of antimicrobial drugs according to sex
Table 8;Comparison of the susceptibility of
microorganism to antimicrobial drugs according to age range
LIST OF FIGURES
Figure 1: Relationship between virulence and
host factors (Adopted from WHO 2000)
Figure 2: Pathogenesis of UTI (Ana et
al., 2015)
Figure 3: Manual dipstick urinalysis (original
picture)
Figure 4: Growth of E. coli on CLED
(Becton Dickinson, 2012)
Figure 5: Sex distribution of the population
Figure 6: Repartition of the population base on
age
TABLE OF CONTENTS
CHAPTER ONE: INTRODUCTION 1
1.1-Background 2
1.2-Statement of problem 3
1.3-Research questions 3
1.4-Research objectives 3
1.5-Hypothesis 3
1.6-Significance of the study 4
CHAPTER TWO: LITERATURE REVIEW 5
2.1-Urinary tract infections (UTIs) 6
2.2-Epidemiology 6
2.3 Types of urinary tract infections 7
2.4-Etiology 7
2.5-Mode of transmission 7
2.6-Physiopathology 8
2.7-Pathogenesis 8
2.8-Risk factors 10
2.9-Clinical manifestation 12
2.10-Laboratory diagnosis 12
2.11-Antimicrobial susceptibility 18
2.12-Treatment 19
2.13- Prevention and control 19
2.14-Previous studies related to the topic 21
CHAPTER THREE: MATERIALS AND METHOD 22
3.1-Study design 23
3.2-Study area 23
3.3-Study duration 24
3.4-Study population 24
3.5-Sample size 24
3.6-Sample method 25
3.7-Selection criteria 25
3.8-Data collection and analysis 25
3.9-Data analysis 25
3.10 -Ethical consideration 26
CHAPTER FOUR: RESULTS AND INTERPRETATIONS 27
4.1 Clinical characteristics 28
4.2 Repartition of the microorganism in the study population
29
4.3 Repartition of the microorganism according to sex 30
4.4 Repartition of microorganisms according to age range 30
4.5 Repartition of microorganism according to their
susceptibility to the class of antimicrobial drugs 32
4.6 Comparison of the susceptibility of antimicrobial drugs to
microorganism according to sex 33
4.7; Comparison of the susceptibility of microorganisms to
antimicrobial drugs according to age range 36
CHAPTER FIVE: DISCUSSION, CONCLUSION AND RECOMMENDATIONS
41
5.1 DISCUSSION 42
5.2-Conclusion 43
5.3-Limitations of the study 43
5.4-Recommendations 44
REFRENCES 45
LIST OF APPENDICES 49
1.1-Background
Urinary tract infection (UTI) is the pathological invasion of
the urinary tract by microorganisms. It poses a major public health problem in
terms of morbidity and financial cost (Oluwafemi et al., 2018). UTI is
considered as one of the most common bacterial infections acquired in the
community and in hospitals (Foxman, 2010).
It may be asymptomatic, acute, chronic and complicated or
uncomplicated, and its clinical manifestations depend on the portion of the
urinary tract involved, the etiologic organisms, the severity of the infection,
and the patient's ability to mount an immune response to it. Both asymptomatic
and symptomatic UTIs possesses a serious threat to public health, hence
reducing the quality of life and resulting into work absenteeism (Otajevwo et
al., 2015). About 50% of women would have experienced symptomatic UTI during
their lifetime while approximately 20% of all UTIs occur in men (Griebling,
2005). The main cause of UTIs are bacteria but other cause can also include,
fungal and viral infections (Wubalem et al., 2017). Females are more
susceptible to this infection than males due to the small and wide size of
their urethra and also hormonal activities (Jane-francis et al.,
2012). The prevalence can also be age and sex dependent (Singh et al.,
2016).
Urinary tract infection is also known to cause short-term
morbidity in terms of fever, dysuria, and lower abdominal pain (LAP) and
complications may result in permanent scarring of the kidney (Griebling,
2005)
The symptoms of UTIs such as fever, burning sensations while
urinating, lower abdominal pains (LAP), itching, formation of blisters and
ulcers in the genital area, genital and suprapubic pain and pyuria generally
depend on the age of the person infected and the location of the urinary tract
infected (Foxman, 2010). The prevalence of UTIs vary from one geographical
location to another. Several factors such as gender, age, race, circumcision,
HIV, diabetes, urinary catheter, genitourinary tract abnormalities, pregnancy,
infants, elderly, and hospitalization status bear signi?cant risk for recurrent
UTIs (Odoki et al., 2019).
The emergence of antimicrobial drugs resistance in the
management of UTIs is a serious public health issue particularly in developing
countries where there is high level of poverty, illiteracy, poor hygienic
practices and drugs of questionable quality (Fagan et al., 2015).
1.2-Statement of problem
A high prevalence of UTIs resulting from illiteracy, poverty
and poor quality of drugs leading to antimicrobial drug resistance, a
short-term morbidity in terms of fever, dysuria and LAP that may lead to a
permanent scarring of the kidney. This situation serve as a motivation to
evaluate the major pathogens causing UTIs among patients attending" The
District Hospitalof Nylon"(DHN) and their susceptibility to common
antimicrobial drugs.
1.3-Research questions
1- What are the most common Uropathogens causing UTIs among
patients attending the DHN?
2- What are the susceptibility pattern of UTI isolates to
common antimicrobial drugs among patients attending DHN?
1.4-Research objectives
1.4.1-Main objective
This study aim at determining, uropathogens causing UTIs and
their susceptibility pattern to common antimicrobial drugs among patients
attending the DHN.
1.4.2-Specific objectives
· To identify the strains of microorganisms causing UTIs
among patients attending the DHN.
· To identify the susceptibility of the isolated
microorganism to common antimicrobial drugs.
1.5-Hypothesis
Ø Null hypothesis
- The most commons uropathogens causing UTI among patients
attending the DHN are bacteria.
- The isolates from patients attending DHN are resistant to
common antimicrobial drugs.
Ø Alternative hypothesis
- The most common uropathogens causing UTIs among patients
attending DHN arenot bacteria.
- The isolates of patients attending DHN are sensitive to
common antimicrobial drugs.
1.6-Significance of the study
To evaluate the microorganisms responsible for UTIs and their
susceptibility to common antimicrobial drugs as a major diagnosis for a
reduction in cases of UTIs and patients resistance to antimicrobial drugs. This
would serve as information to policy makers in order to guide the health
workers on the necessity of creating awareness about the modes of prevention of
these infections and the necessity of antimicrobial tests before
prescriptions.
2.1-Urinary tract infections (UTIs)
UTIs is a term that describes infection resulting from
invasion of the urinary tract by microorganisms. This may be caused by
bacteria, fungi, protozoans or viruses with the bacteria been the most invasive
microorganism. This is due to their virulence factors, their adaptive capacity
but also the susceptibility of the host (Oluwafemi et al.,2018;
Gachuhi,2017).
It is considered as one of the most common bacterial
infections acquired in the community and hospitals with a range of symptoms
which generally depends on the age, sex and the infected location of the
urinary tract of the infected person (Foxman, 2010). Females are usually more
susceptible to this infections than males due to the short and wide size of
their urethra, absence of prostatic secretions and hormonal changes resulting
from pregnancy(Singh et al.,2016). It is the common cause of acute
illness in infants and children less than five worldwide accounting for the
heaviest burden disease (Dorgelesse et al.,2019).
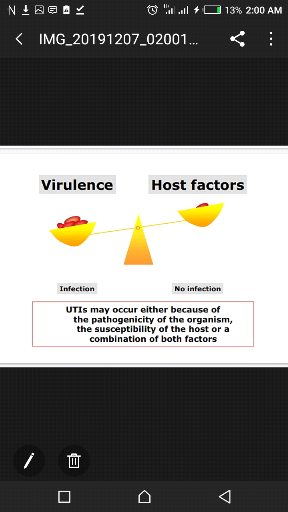
Figure 1:Relationship between virulence and host
factors (Adopted from WHO 2000)
2.2-Epidemiology
UTIs are one of the most common microbial diseases encountered
in medical practices. Worldwide, it has an estimated prevalence of around 150
million persons per year (May et al.,2016). It is a considerable
health problem which ranks as the second leading cause of infections after the
respiratory tractinfections. Healthcare-associated urogenital tract infections
(HAUTI) are some of the most-frequently occurring health associated infections
thatconstitute 19.0% according to the European point prevalence survey (Rahimi
et al.,2018; Wagenlehneret al.,2016). It is also considered
as one of the severe public health problem imposing a high morbidity and
mortality rate as well as severe economic consequences worldwide (Gemzu et
al.,2016).
All individuals are susceptible to UTIs; however the
prevalence very with age, sex and certain predisposing factors. Among the,
urinary tract infections (UTIs) are most common encountered diseases by
clinicians in developing countries with an estimated annual global incidence of
at least 8.3 million doctor visit yearly (Annuli et al.,2016).
UTI bacteria are often from fecal origin, and anaerobic
bacteria rarely cause UTI. Among this bacteria, 90% are E. coli,
10-20% Staphylococcus saprophyticus and 5% is caused by
Enterobacter. Young sexually active females are the most prevalent
according to(Emiru et al., 2013)
2.3 Types of urinary tract infections
Urinary tract infection usually develops in the lower urinary
tract (urethra and bladder) and if not properly treated they ascend to the
upper urinary tract (ureters and kidneys) and may cause severe kidney
damages.The diseases are bladder infection (cystitis), urethra
infection(urethritis), andkidney infection (pyelonephritis).
2.4-Etiology
The major cause of UTIs are bacteria, with the most common
agents been from the Enterobacteriaceae family that is (E.coli ,
Klebsiella spp, Proteus spp, Serratia spp, Enterobacter
spp,Pseudomonas spp) with E coli been the most prevalent. Others
include: S saprophyticus,E. faecalis, S. agalactiae, S. pyogenes ,
S.aureus.Gram negative bacteria account for 90% while gram positive have
only 10%.Other causes of UTIs include: parasites, fungi and viruses (Wubalem
et al.,2017). Also etiology varies depending on health status,
residential status(institutionalized or not), age, history of current
catheterization, spinal cord dysfunction, history of antimicrobial drugs,
sexual activities, type of pants used, type of toilet (Martha and Edgardo,
2019).
2.5-Mode of transmission
UTIs can be gotten from sexually transmitted infection, injury
from an instrument such as urinary catheter,an exposure to an irritating
chemical substances such as antiseptic or spermicide, bacteria resulting from
fecal pathogens, exchanging under wears, using chemical substances for vaginal
douching and the type of toilet (Annuli et al.,2016).
2.6-Physiopathology
2.6.1-Anatomy and physiology of the urinary
system
The urinary system consists of the kidneys, ureters, urinary
bladder, and urethra. Often, urinary tract infections (UTIs) are characterized
as being either upper or lower based primarily on the anatomical location of
the infection. The lower urinary tract encompasses the bladder and urethra,
while the upper urinary tract encompasses the kidneys and the ureters. The
kidneys filter the blood to remove wastes and produce urine. The ureters,
urinary bladder, and urethra together form the urinary tract, which acts as a
plumbing system to drain urine from the kidneys, store it, and then release it
during micturition. Besides filtering and eliminating wastes from the body, the
urinary system also maintains the homeostasis and blood pressure (Annuli et
al.,2016)
2.6.2-Pathology
UTI are amongst the most common bacterial infections. They
occur either as an uncomplicated host setting characterize by no underlying
structural or functional abnormality in the patients genitourinary tract, or
complicated characterize by clinical manifestations. The two major predisposing
factors are the presence of a foreign body like the urinary catheter and a
disruption in normal urine flow as a result of obstruction or retention. The
presence of urinary catheter or any other urine drainage device leads to the
developments of a biofilm which in turn shields them from being eradicated
completely (Walsh and Collyns,2017). Initially, about 95% of UTI occur when
bacteria ascend the urethra to the bladder, and in cases of pyelonephritis
ascend the ureter to the kidney.The remainder of UTIs are hematogenous
(Imam,2018).
2.7-Pathogenesis
Adherence is a key event initiating each step in UTI
pathogenesis. A UTI typically starts with periurethral contamination by a
uropathogen, followed by colonization of the urethra and subsequent migration
of the pathogen to the bladder. The eventrequires appendages such as flagella
and pilli. In the bladder, the consequences of complex host pathogen
interactions ultimately determine whether uropathogens are successful in
colonization or eliminated.Multiple bacterial adhesins recognize receptors on
the bladder epithelium (also known as the uroepithelium) and mediate
colonization. Uropathogens such as UPEC
(Uropathogenic E.coli) survive by invading the
bladder epithelium, producing toxins and proteases to release nutrients from
the host cells, and synthesizing siderophores to obtain iron(Ana et al.,
2015). By multiplying and overcoming host immune surveillance, the
uropathogens can subsequently ascend to the kidneys, again attaching via
adhesins or pili to colonize the renal epithelium and then producing
tissue-damaging toxins. Consequently, the uropathogens are able to cross the
tubular epithelial barrier to access the blood stream, initiating bacteremia.
Also, uropathogens often form biofilms that are responsible for colonization
and persistence leading to drug resistance. Catheterization also brings in
uropathogens which developpe a biofilm that adhere, colonize and persist in
causing UTI (Ana et al.,2015).
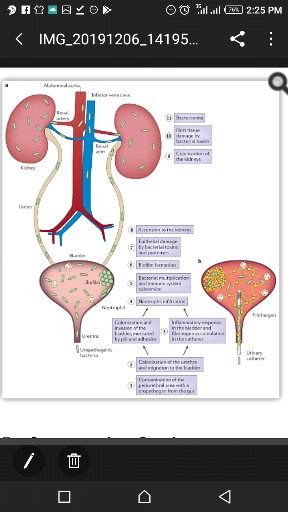
Figure 2:Pathogenesis of UTI (Ana et
al.,2015)
2.8-Risk factors
The urinary system is biologically structured to help ward
off infections. The ureters and bladder are supposed to prevent urine from
backing up towards the kidneys. The flow of urine from the bladder is designed
to wash bacterial out of the body. Despite all these, infections still occurs
due to some factors such as:
Ø Alterations to the host's defense
mechanisms
The host natural flora is usually altered due to actions such
as extreme use of antimicrobial agent, use of contraceptives like spermicide
and obstruction of urine flow.
Also illness such as diabetes mellitus, HIV infection and
other diseases impacting the immune system and kidney.
Ø Anatomical and Physiological
Factors
Anatomical and physiological factors contribute to a greater
prevalence of UTIs in females compared to males. Female pelvic anatomy plays an
important predisposing role for recurrent UTIs. A study carried by Hooton
et al. (2010) investigated differences in perianal anatomical
measurements and discharge characteristics in 100 females with a history of
recurrent UTIs and in 113 females with no prior history of UTIs. Analysis of
the results demonstrated that the urethra and anus were significantly closer
together in cases of UTI (4.8 #177; 0.6cm) compared to controls (5.0 #177;
0.7cm). Other important physiological and anatomical factors that predispose
tobacterial adherence in females (compared to males) include a shorter urethra
and the absence of antibacterial properties provided by prostatic fluid.
Ø Premenopausal / Menopausal
Females
In premenopausal women, 90% of the vaginal flora is
Lactobacilli, which protect the system against colonization with
uropathogens such as E. coli, with estrogen loss at menopause, it
results in the thinning of the vaginal epithelium and decreased amount of
glycogen. The resulting environment is usually hostile to Lactobacilli
thereby decreasing their numbers. Biological changes due to menopause put
these women at particular risk of contracting both primary and recurring UTIs
because with estrogen loss, the walls of the urinary tract becomes weak and as
such it reduces its ability to resist bacteria colonization (Nicolle, 2008).
Ø Age and Sex
The incidence of urinary tract infection increases with age.
During the first few months of life, the incidence of urinary tract infections
in male exceeds that of females. From the first year onwards, both first time
and recurrent urinary tract infection is much more common in females. The
female urethra appears to be particularly prone to colonization because of its
proximity to the anus.Men's risk for UTI increases with age, men become more
susceptible to UTIs after 50 years of age, when they are more likely to develop
prostate problems due to loss of prostate fluid. Enlarged prostate gland can
also impede and slow the flow of urine, thus raising the risk of infection.
Nicolle, (2008) observed that men who are not circumcised tend to also be more
prone to developing UTIs because these bacterial build up much more easily in
the folds of the extra skin on the penis thereby making them more susceptible
to developing UTIs.
Ø Obstruction
Obstruction to the flow of urine from the kidney through the
pelvis, ureter, bladder, and urethra, is a common disorder. It causes a rise in
pressure within urinary tract, which predispose to urinary tract infection.
Obstruction may occur at any level but is most often found at the pelvis
ureteric junction. Obstruction to the easy flow of urine may be the result of
some gross anatomical abnormalities such as congenital or acquired pathological
conditions in the urinary tract. Obstruction can also lead to reflux of
infected urine in the urethra back into the ureter and kidney with consequent
pyelonephritis.
Ø Instrumentation
Bacteria develop in at least 10-15% of hospitalized patients
with indwelling urethral catheters. Factors associated with an increased risk
of catheter associated urinary tract infection include, prolonged
catheterization, severe underlying illness, disconnection of the catheter and
drainage tube and lack of systemic antimicrobial therapy. Bacteria usually
enter the catheter system at the catheter collecting tube junction or at the
drainage bag portal. The organisms then ascend into the bladder within causing
annoying symptoms.
Ø Management of urinary tract
infections
Management of urinary tract infections typically involves drug
therapy and patients' education. The ideal treatment of urinary tract infection
is an antimicrobial agent that effectively eradicates bacteria from the urinary
tract with minimal effects on fecal and vaginal flora, thereby minimizing the
incidence of vaginal yeast infections. The antibacterial agent used for the
management of uropathogensshould be affordable, produce few side effects and
oflow resistance. Various treatments regimen have been used successfully to
treat uncomplicated lower urinary tract infections in women.Early recognition
of urinary tract infection and prompt treatment are essential to prevent
recurrent infection and complications such as renal failure and sepsis (Annuli
et al.,2016).
2.9-Clinical manifestation
UTIs can either be symptomatic or asymptomatic characterized
by a wide spectrum of signs and symptoms depending on the part of the urinary
tract infected. Infections may either involve only the lower part of the
urinary tract or both the lower and upper part. This may include
cystitis,pyelonephritis,fever, chills, nausea, vomiting, and diarrhea. Also, in
about 30% of cases patient urine become cloudy malodorous and bloody. White
blood cells and bacteria can be detected by examination in the urines of an
infected person. Pyelonephritis can be determined by bacteremia that is
bacteria in blood (Gachuhi,2010; Khoshbakht et al., 2013).
2.10-Laboratory diagnosis
Evaluation of UTI relies on both laboratory analysis and
clinical manifestations.Laboratory analysis for UTIs can be done in four ways:
dipstick urinalysis, microscopic urinalysis, urine culture and molecular
identification (Christineet al.,2018). Technic for specimen collection
is important in order to avoid contaminants (Gachuhi,2010)
2.10.1-Specimen Collection:
In adults, most urine specimens for laboratory examination are
obtained by the clean catch-voided midstream technique. This technique is
widely accepted and applied because it is simple, inexpensive and non-invasive
and there is no risk of complications. Colony counts from urine specimens
collected by this method correlate reasonably well compared with those of
specimens collected by supra-pubic aspiration or straight catheterization. A
disadvantage of this technique is that the urine can be contaminated with
commensal bacteria during its passage through the distal urethra. Simple
procedures to decrease contamination rate include cleaning of skin and
mucousmembranes adjacent to the urethral before micturition and the collection
of the midstream part of the urine.
Proper collection ofsamples by this method may be problematic
in young children, elderly and disabled patients. Supra-pubic aspiration is the
best method to avoid urethral contamination, especially in young children. But
it is infrequently used because it is invasive, uncomfortable and
time-consuming. Collection of urine by use of a single catheter (straight
catheter technique) is the next-best technique to obtain urine specimens with
minimal contamination risk. However, the technique is not widely applied
because of several disadvantages: it is labor intensive, costly and invasive.
By the insertion of the catheter through the urethra, bacteria can be forced
into the bladder, which involves a risk of infection.For babies one method is
to place a specially designed absorbent pad in a nappy (supplied by a doctor).
Urine is sucked into a syringe from the wet pad. Another method is to use a
plastic bag that sticks on to the skin and collects urine
Because laboratory procedures for urine cultures depend upon
the type of urine specimen, it is indispensable that the collection method is
specified on the laboratory request form. Some essential information includes
date and time of specimen collection and any clinically relevantinformation
(e.g. antimicrobial treatment, predisposing urological conditions such as
anatomic abnormalities, stones or the presence of foreign material) (Gachuhi,
2010;Oyaert et al., 2018).
2.10.2-Macroscopy:
The report on the appearance (that is the colour, odour and the
clearance or turbidity) of the urine collected is done by eye observation. From
it, some possible causes might be suspected. For UTIs (Cheesbough, 2006).
Table 1; Macroscopy of urine and its
implication
|
Appearance
|
Possible Causes
|
|
· Cloudy urine usually with an unpleasant smell
|
Bacterial infection
|
|
· Red and cloudy urine
|
Bacterial infection
|
2.10.3-Dipstick urinalysis:
Urine testing often begins with dipstick urinalysis, which is
easily available in laboratories and takes minutes for interpretation.The most
common type of dipstick urinalysis permits analysis of multiple urine
components, the most important being leukocyte esterase (LE), nitrite, and red
blood cells. LE is expressed in white blood cells (WBCs), which are elevated in
urine during infection. Dipstick testing is fairly sensitive to LE in the urine
and turns positive in the presence of (>5-15 WBC/ high-power field (hpf)).
Nitrite is indicative of the presence of bacteria, as some uropathogens
containing bacterial enzymes that convert nitrates into nitrites. Urine
dipsticks are able to detect nitrites in the presence of bacteria (>105
colonies forming unit CFU/mL). Urine dipsticks can detect very low levels of
blood in the urine (correlates with >1-4 red blood cells/hpf).Although blood
may be associated with other pathology, in the presence of symptoms or positive
nitrite and LE testing, its presence may increase the probability of UTI.
Several conditions can influence the interpretation of dipstick urinalysis.
Uropathogens such as Enterococci and Staphylococcus
Saprophyticus do not reduce nitrates and would result in false negatives.
Although testing for nitrites and red blood cells requires only 1 minute before
interpretation, LE requires 2 minutes for accurate interpretation. Urine that
is too dilute may result in lysis of cells, increasing the risk of
false-negative results. Lastly, urine dipsticks cannot distinguish between
myoglobin and hemoglobin, so hematuria based on dipstick urinalysis should
always be checked with microscopic urinalysis (Christine et al.,
2018). The various techniques for urinalysis include;
a) Automated screening systems
Automated screening systems are used for a large output with
minimal labour and a rapid turn-around time compared with conventional
cultures. These methods are expensive and often these costs can be justified
only in laboratories that receive many samples. Several automated urine
screening systems are either bacterial growth independent or dependent. By
examining images of un-centrifuged urine samples using a video camera one is
able to recognize many cellular structures, including leucocytes, erythrocytes,
epithelial cells and microorganisms. A robotic instrument has been introduced
for urine screening using fluorescent stain probes to detect bacterial membrane
from urine sample. After staining, the membrane is examined using fluorescent
microscopy imaging technology to detect the presence of uropathogens in urine.
Although this method is faster there is a need to culture negative urine
specimens to eliminate few organisms which may not have been detected by this
method .The Coral UTI Screen system uses a somatic-cell which releases the
adenosine triphosphate (ATP). On the contrary, in bacterial cells the bacterial
ATP remains protected within the bacterial cell. This then is liberated and
detected by the instrument which is directly proportional to number of present
bacteria. This test has a sensitivity of 86 % and specificity of 76 %
(Gachuhi,2010)
b) Semi-automated technique
Like the automated screening system this technique is used for
large output with minimal labour and a rapid turn-around time. Combi 11-test M
strips on a Miditron-M semi-automate reflectance photometer (Roche diagnostic
Gmbh,Mannheim,Germany) have been use in our routine laboratory for this
analysis.The strips include reagent pads for the semi quantitative assessment
of nitrite, leukocyte esterase, pH, specific gravity, protein, glucose,
ketones, urobilinogen, bilirubin and blood. For analysis a strip is simply
immersed in an un-centrifuged urine sample and placed on the miditron-M
semi-automate reflectance photometer which measures the various parameters and
reads on the screen (Ramazan et al.,2014)
c-) -Manual method
In this condition, analysis is simply done by immersing a
combur 11-test M strip in un-centrifuge urine allowing to stand for 1-2 minutes
and comparing the colour change on the strip with that on the container of the
strips and reporting the values.
Figure 3:Manual dipstick urinalysis (original
picture)
2.10.4-Microscopy
a) Wet mount preparation
Microscopic urinalysis is performed with a manual or automated
light microscope. For this preparation a drop of the sediment of centrifuge
urine is place on a slide and covered with a cover slide then observed on a
manual microscope at objective 10X or 40X with the condenser down and the iris
closed.The presence of leukocytes (pyuria, defined as >5-10 leukocytes/hpf)
or bacteria (bacteriuria,>15 bacteria/hpf) in the urine can be helpful in
diagnosingUTI. Occasionally, hematuria in the presence of bacteriuria or
pyuriamay also indicate UTI. The presence of squamous epithelial cells may
occasionally indicate contamination, and WBC casts may indicate upper urinary
tract inflammation or infection (Chu and Lowder,2018)
b)-Dry mount preparation
After observing bacteria or white cells in the wet mount
preparation amicroscopic examination is performed again by preparing a gram
stain of the urine that will indicate the morphology of the organism, viewed
under a light microscope at objective 100X with the condenser up and the iris
open. The presence of one organism per oil-immersion field in a centrifuged
sample correlates with 100,000 bacteria/ml. White blood cells > 10 WBC/mm3
it only signifies the presence of inflammation. Sterile pyuria is associated
with urinary tuberculosis, chlamydial, and fungal infections. Hematuria,
non-specific, may indicate other disorders such as calculi or tumor.
Proteinuria is found in the presence of infection.
2.10.5- Urine Culture
After observing bacteria in the gram stain, cells,
casts,protein, nitrite or urine with markedly alkaline or acid reaction
culturing is next. It is defined by the presence of more than 105 colony
forming units (CFUs/ml) of single bacteria in cultured urine
(cheesebrough,2006).Normally culture ofnon-invasivespecimens should allowthe
detection of 104 or 105 CFU/mL. This detection is usually
accomplishedby inoculation of 50 ìL of urine onto appropriate media. For
more invasively collected specimens (i.e. supra-pubic aspirations)or for the
culture of yeasts, 100 ìL of urine should be culturedon appropriate
media (Sabouraud Dextrose Agar) to achieve a detection limit of 102
CFU/mL. Inoculationof an additional routine 1 ìL sample can facilitate
interpretationof heavily grown culture media.
Urinary specimens can be inoculated by stricking a quantity of
the sediment of centrifuged urine on a culture media and incubating under
stated conditions. Unless calibrated pipettes are used, colony counts are only
approximations and can be deranged by as much as a hundred-fold. The delivered
isolated colonies can be used for identification andsusceptibility testing
especially at higher counts. One colony does not represent one CFU, nor is this
accuracy necessary for urine culturing. Due to the several practical
advantages,it is suggested to use sterile, calibrated and disposable or
automated 1 and 10 ìL loops for inoculation of urinary specimens.
Besides CLED (Cysteine lactose electrolyte deficient) agar,
which is the best culture media of choice for urine pathogens as stated by
(Cheesbough, 2006), a variety of chromogenic selective media are available for
the identification and differentiation of urine pathogens. These
chromogenicmedia can be used for all urine specimens or those that might be
considered to be at a higher risk for contamination. Specific organisms will
produce colored colonies, depending upon interaction between the enzymes they
produce and the substrates incorporated into the medium, allowing direct
identification of the most relevant urinary Enterobacteriaceae and Enterococci.
In addition to CLED or chromogenic media, a more universal blood agar plate
could be inoculated allowing the detection of Gram-positive and fastidious
bacteria(Oyaert et al.,2018).
On the CLED media, culture is by inoculating
a loopful of urine carried with a sterile calibrated wire loop, strick on a
plate of CLED media and incubating the plate aerobically at 35-37°C
(Cheesbough,2006).
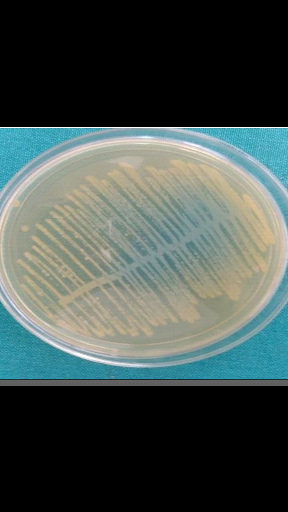
Figure 4:Growth of E.coli on CLED
(Becton Dickinson, 2012)
After culture on any of the stated media the bacterial
isolates are further characterized using standard microbiology techniques such
as colony morphology, Gram-staining, catalase test and other biochemical tests
which include oxidase, Kia, indole, citrate utilization, H2S
production Voges-Proskauer, methyl red, urease and sugar fermentation testes
(Ndamason et al., 2019)
2.10.6-Molecular identifications of
uropathogens
v Polymerase chain reaction (PCR)
Urine samples are collected from UTI patients with clean catch
midstream technique. The samples are centrifuged and cotton swabs used for
inoculation of brain heart infusion broth. The media then incubated for 3h at
37°C and the culture saved in refrigerator for deoxyribonucleic acid (DNA)
extraction purpose for analysis (Ibraheam et al., 2016).
v Lateral flow immunosorbent assay
Lateral flow assays are a good choice for point-of-care
screening tests; they are inexpensive and easy to use, as the sample and
reagents are mixed on a paper support with liquid transport driven by capillary
action and a colorimetric readout. Dipstick tests for urine nitrite and
leukocyte esterase are widely used for lateral flow assays, but they are
limited by shortcomings of poor sensitivity (Davenport et
al.,2017).
v Flow cytometry
It is a rapid screening based on the detection of cells in
solution by light scattering.It has been employed in many devices and can
detect most bacterial species as well as fungi. Flow cytometry systems, uses a
combination of light scattering and fluorescence to rapidly screen for the
presence of bacteria in urine. Flow cytometry is a good system for selecting
samples for further analysis, and has been used to identify pathogen-positive
urine for further complex testing, such as mass spectrometry analysis. Initial
screening of urine samples by flow cytometry might improve clinical laboratory
workflow by reducing the number of samples sent for further analysis; however,
flow cytometry is only a screen for bacteriuria as it does not provide species
identification(Davenport et al.,2017).
2.11-Antimicrobial susceptibility
The aim of the laboratory in the management of UTI is for
accurate and timely diagnosis with appropriate antimicrobial susceptibility
testing. However global data shows an increasing multidrug resistance among
uropathogens to conventional drugs. Some factors favoring this antimicrobial
resistance are; mutations, exposure to cells with new genetic material and use
of antimicrobial agents as growth promoters in animal feeds destined for human
consumption give rise to multidrug resistance. However, the misuse of
antimicrobial agents resulting from the mal-administration of antimicrobial
drugs, incorrect use of antibiotics for the prophylaxis of recurrent UTIs,
self-medication and use of drugs over the counter without prescription of the
clinicians has highly contributed to antimicrobial resistance.
A diagnosis, along with early initiation of appropriate
antimicrobial drug therapy would have a potential to minimize the risk of a
poor outcome, reduces chronicity & drug resistance thus decreasing
patient's sufferings and financial expenditure (Gachuhi,2017; Parvee et
al.,2015).
2.12-Treatment
A spectrum of antimicrobial drugs exist for the treatment of
UTIs but a good treatment should be used when culture results become available
to avoid drug resistance therefore antimicrobial sensitivity test should be
used to direct therapy. Management of uncomplicated UTIs should be done on two
important principle organisms especially E.coli which accounts for
more than half of all urinary isolates and Staphylococcus saprophyticus
which accounts for less than a quarter of the urinary isolates. Nosocomial
and uncomplicated community acquired UTIs rate the highest in antimicrobial
drugs resistance. In the treatment of UTIs some of the following antibiotics
may be recommended for cases of bacterial infections; ciprofloxacin, ofloxacin
and ceftriazones. Their efficiency is seen when given for 3 days to treat acute
symptomatic and uncomplicated lower urinary tract infections (Gachuhi,2017)
2.13- Prevention and control
The following practices are recommended to promote overall
urinary health, thereby reducing or preventing the occurrence of UTIs.
v Ensure proper hydration and nutrition
Dehydration results in concentrated urine and less frequent
voiding, conditions that support bacterial growth in the bladder. Dehydration
is a concern for residents who may also be on medications that increase
diuresis or who have a disease such as diabetes that may cause excessive
urination. Adequate hydration is indicated by pale-coloured urine, moist mucous
membranes, and/or normal specific gravity of the urine. The following
strategies may be used to promote adequate hydration in residents:
· Offer a variety of fluids throughout the day.
· Routinely encourage fluid intake during social activities
such as «Happy Hour» or «Tea Time», as well as in
therapeutic group activities.
· Offer foods that contain high water content.
· Educate residents, healthcare providers, and families on
the importance of hydration and urinary health.
· Document the resident's preference for type and
temperature of fluids, and customize a plan that will best meet the hydration
needs of the client.
· Maintain therapeutic blood glucose levels in residents
with diabetes.
v Provide good perianal hygiene
· Ensure that personal hygiene is performed correctly to
prevent prolonged contact with urine or feces.
· Perineal hygiene with mild soap and water should be done
daily, and after episodes of bowel incontinence.
· Women should avoid cleaning the anus from up to down.
v Promote healthy voiding habits
· Completely emptying the bladder is best accomplished by
providing a relaxed voiding environment with a comfortable toilet seat at the
appropriate height and convenient safety hand rails.
· Ensure that any issues with constipation or fecal
impaction are addressed (Valerie .,2013)
2.14-Previous related studies on the topic
Table 2: previous study related to the topic
|
Authors/
Years
|
Title of the studies
|
Sample size
|
Prevalence
|
|
Akoachera et al.,2012
|
Etiologic profile and antimicrobial susceptibility of community
acquired UTIs in two Cameroonian towns (Buea and Bamenda).
|
Buea=85
Bamenda=150
|
65.9%
54%
|
|
Wubalem et al., 2018
|
Prevalence and antibiotic susceptibility of uropathogens from
cases UTIs in Shashemene referral hospital Ethiopia
|
384
|
88.5%
|
|
Rahimi et al., 2018
|
Antimicrobial resistance profile of UTIs at a secondary care
hospital in Median Indonesia
|
96
|
88.32%
|
|
Ke he et al., 2019
|
Prevalence , risk factors and microorganism of UTIs in type 2
diabetes mellitus; a retrospective study in china
|
3652
|
11.19%
|
|
Gachuhi, 2017
|
Antibiotic susceptibility pattern of bacterial uropathogens
isolated from patients of Nakuru level 5 hospital Kenya
|
385
|
29.0%
|
3.1-Study design
This study was a cross sectional retrospective study
design.
3.2-Study area
3.2.1-Presentation of the study area
DHN is a hospital enclosed by a fence with three sky blue
gates and 4 main buildings painted in sky blue and white within possessing
sixteen services,
3.2.1.1-History and origin of the area
DHN was brought about from the transformation of the
progressive former Infant and maternal protection (IMP) Tergal to a maternity
then an integrated health center (IHC). It was inaugurated as the DHN on the
24/09/1999 by professor Monekosso minister of public health
3.2.1.2-Geographical location of the area
DHN is a district hospital located in the littoral region of
Cameroon, in the Wouri division at the sub-division of Douala III, more
precisely in the quarter whose name is carried by the hospital. It is 200metres
from the main road limited to the north by stores, to the west by a craft shop,
east by stores and to the south by Chococam industry.
3.2.2-Structural organization
The structural organization of DHN is as
follow;
· The director
· Management committee
· The Secretary
· The burser
· The Accountant
· The recipe manager
· The superintendent
· The chief service of medicine
· The chief service of pediatrician
· The chief service of surgical department
· The chief service of maternity
· The chief service of emergency
· The focal point of UPEC
· The major of surgical department
· The major of medicine
· The major of UPEC
· The major of pediatrician
· The major of mortuary
· The major of cardiologist
· The major of CPN
· The major of emergency
3.2.3-Reasons for choosing the place of
study
DHN is a hospital that falls under the secondary stage of
prevention of the pyramid of prevention. This stage has to do with people at
the onset of health problems and the measures of prevention here include; early
screening, intervention and control of risks factors which are the objectives
of my study.
3.3-Study duration
This study was carried out within a period of 5 days that is
from the 27th - 31st December 2019.
3.4-Study population
The study targets both male and female out patients and
inpatients of all age groups with diagnosed cases of UTIs from November 2019 to
June 2019.
3.5-Sample size
The minimum sample size for this study was calculated
according to the method described by Daniel in 1999.
n=t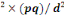 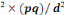
where;
n=sample size
t=95% confidence interval (1.96)
p=past prevalence of UTI
q=1-p
d=margin error (0.05)
Taking the prevalence of UTIs in Ethiopia proven to be 88.5%
in 2018(Wubalem et al.,2018)
n= (1.96)²×0.885(1-0.885)/(0.05)²
=162 (An estimation)
A total of 248 patients were collected.
3.6-Sample method
A simple random technique was used in this study.
3.7-Selection criteria
3.7.1-Inclusion criteria
All patients attending DHN diagnosed with UTI and
susceptibility pattern to drugs were included in the study.
3.7.2-Exclusion criteria
All patients attending DHN not diagnosed with UTI and
susceptibility pattern to drugs.
3.8-Data collection and analysis
3.8.1 Method of sample collection
Data were collected from laboratory registers. A copy of
consent from the administration were been given to the laboratory technician
and analysis were made from the date collected in registers. From that analysis
results were obtained and statistic drawn from it and given in percentage,
tables and charts.
3.8.2 Method of specimen analysis
During the period of collection specimen were collected from
patients who presented clinical manifestations of UTIs and analyzed practically
by three methods;
· The automated strip analysis method using COMBI 11
strip
· Macroscopy and microscopy
· Culture on CLED,EMB (ethylene methylene blue) and
SABOURAUD AGARS.
Antimicrobial drugs susceptibility were analyzed using the
Mueller Hilton agar.
3.9-Data analysis
Data collected or obtained from the laboratory registers were
inserted in an Excel sheet then transfer to SPSS version 20 for analysis.
The Chi square test was used to compare variable whereas the
logistic regression analyses was used the association.
3.10 -Ethical consideration
· An authorization from the ministry of higher education.
· An authorization from the school and hospital
concerned.
· Plagiarism will be avoided.
· All informations collected from the register are kept
strictly confidential.4.1 Clinical characteristics
Of a total of 248 samples that was included in our study,
79.03%(196/248) were from females whereas 20.97%(52/248) from males.
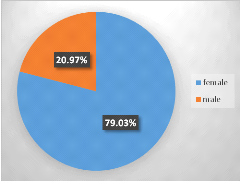
Figure 5: Sex distribution of the population
The mean age of the population was 29.15 (19-40) years with
the mean age of the female population being35.28 #177; 1,26 years while for
male it was 28.37 #177; 3,21 years with significant difference (P= 0,044)
observe between the two means groups. The variation of age was between 1 and 79
years.
The repartition of the study population according to the age
shows that patients of age between 21-30 and 1-10 with the respective
percentages of 29.84% and 16.53% were more prevalent while patients of 71-80
been less represented (0.40%). Significant difference (P =0.0001) was observein
the repartition of the population according to age.
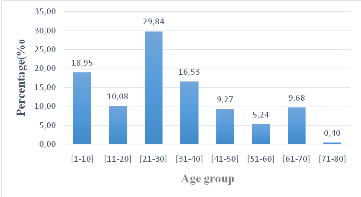
Figure 6: Repartition of the population base
on age
4.2 Repartition of the microorganism in the study
population
Significant difference (P<0.0001) was observe in the
infectious repartition of microorganisms with E.coli been the most
infectious UTIs microorganism (31.45%) follow by Staphyloccocus Sp
(27.02). The multiple infection was common with E coli and
Candidas sp (2.42%).
Table 3: Repartition of the microorganism in the study
population
|
MicroorganismsNumberpercentagesp-value
C. albicans
124.84
<0.0001
E. coli
7831.45
Klebsiella sp.
3514.11
Proteus sp.
2610.48
P. stuartii
114.44
P. aeruginosa
31.21
Serratia sp.
10.40
Staphylococcus sp.
6727.02
Streptococcus sp.
20.81
E. coli + Candida sp.
62.42
Klebsiella sp. + Candida sp.
31.21
Staphylococcus sp. + Candida sp.
31.21
Streptococcus sp. + Candida sp.
10.40
|
4.3 Repartition of the microorganismaccording to
sex
The reparation of microorganism according to sex show
significant difference in the variation between both sexes with males more
infected withKlebsiella Sp, Proteus Sp, Staphyloccocus
SP, while female was more infected with E. Coli, P
Stuatii and C. albicans. No significant difference was observe in
the infection between male and female in Serratia Sp,
Streptococcus Sp, and also in all multiple infection.
Table 4: Repartition of the microorganism according to
sex
|
|
Female
|
Male
|
p-value
|
|
Microorganisms
|
Number
|
percentages
|
Number
|
Percentages
|
|
C. albicans
|
12
|
6.12
|
0
|
0.00
|
0.0007
|
|
E. coli
|
67
|
34.18
|
11
|
21.15
|
<0.0001
|
|
Klebsiella sp.
|
18
|
9.18
|
17
|
32.69
|
>0.999
|
|
Proteus sp.
|
19
|
9.69
|
7
|
13.46
|
0.038
|
|
P. stuartii
|
10
|
5.10
|
1
|
1.92
|
0.017
|
|
P. aeruginosa
|
3
|
1.53
|
0
|
0.00
|
0.375
|
|
Serratia sp.
|
1
|
0.51
|
0
|
0.00
|
>0.999
|
|
Staphylococcus sp.
|
52
|
26.53
|
15
|
28.85Sta
|
<0.0001
|
|
Streptococcus sp.
|
2
|
1.02
|
0
|
0.00
|
0.75
|
|
E. coli + Candida sp.
|
5
|
2.55
|
1
|
1.92
|
0.3125
|
|
Klebsiella sp. + Candida sp.
|
3
|
1.53
|
0
|
0.00
|
0.375
|
|
Staphylococcus sp. + Candida sp.
|
3
|
1.53
|
0
|
0.00
|
0.375
|
|
Streptococcus sp. + Candida sp.
|
1
|
0.51
|
0
|
0.00
|
>0.999
|
4.4 Repartition of microorganisms according to age
range
Significant difference was observe in the repartition of
microorganism according to age range with C. albicans, E. coli, Klebsiella
sp, Proteus sp, P. stuartii, and Staphylococcus sp, with E
coli been the most abundant in majority of the age groups. High prevalence
was observe in age range of [31-40] (9.76%), [41-50] (56.52%), [11-20]
(32.00%), [51-60] (15.38%), [11-20] (10.64%) [21-30] (41.89%) respectively to
microorganism
Table 5: Repartition of microorganisms according
to age range
|
|
[1-10]
|
[11-20]
|
[21-30]
|
[31-40]
|
[41-50]
|
[51-60]
|
[61-80]
|
p-value
|
|
Microorganisms
|
Number
|
percentages
|
Number
|
percentages
|
Number
|
percentages
|
Number
|
percentages
|
Number
|
percentages
|
Number
|
Percentages
|
Number
|
percentages
|
|
C. albicans
|
3
|
6.38
|
0
|
0.00
|
5
|
6.76
|
4
|
9.76
|
0
|
0.00
|
0
|
0.00
|
0
|
0.00
|
0.0087
|
|
E. coli
|
20
|
42.55
|
4
|
16.00
|
14
|
18.92
|
11
|
26.83
|
13
|
56.52
|
7
|
53.85
|
9
|
36.00
|
0.0235
|
|
Klebsiella sp.
|
6
|
12.77
|
8
|
32.00
|
9
|
12.16
|
2
|
4.88
|
3
|
13.04
|
0
|
0.00
|
7
|
28.00
|
0.0344
|
|
Proteus sp.
|
3
|
6.38
|
2
|
8.00
|
11
|
14.86
|
5
|
12.20
|
1
|
4.35
|
2
|
15.38
|
2
|
8.00
|
0.0038
|
|
P. stuartii
|
5
|
10.64
|
2
|
8.00
|
1
|
1.35
|
2
|
4.88
|
0
|
0.00
|
0
|
0.00
|
1
|
4.00
|
0.0803
|
|
P. aeruginosa
|
0
|
0.00
|
1
|
4.00
|
0
|
0.00
|
0
|
0.00
|
1
|
4.35
|
1
|
7.69
|
0
|
0.00
|
0.6767
|
|
Serratia sp.
|
0
|
0.00
|
0
|
0.00
|
0
|
0.00
|
1
|
2.44
|
0
|
0.00
|
0
|
0.00
|
0
|
0.00
|
0.4232
|
|
Staphylococcus sp.
|
7
|
14.89
|
6
|
24.00
|
31
|
41.89
|
11
|
26.83
|
5
|
21.74
|
3
|
23.08
|
5
|
20.00
|
<0.0001
|
|
Steptococcus sp.
|
0
|
0.00
|
0
|
0.00
|
0
|
0.00
|
1
|
2.44
|
0
|
0.00
|
0
|
0.00
|
0
|
0.00
|
0.4232
|
|
E. coli + Candida sp.
|
2
|
4.26
|
1
|
4.00
|
1
|
1.35
|
2
|
4.88
|
0
|
0.00
|
0
|
0.00
|
0
|
0.00
|
0.4616
|
|
Klebsiella sp. + Candida sp.
|
1
|
2.13
|
1
|
4.00
|
0
|
0.00
|
1
|
2.44
|
0
|
0.00
|
0
|
0.00
|
0
|
0.00
|
0.6767
|
|
Staphylococcus sp. + Candida sp.
|
0
|
0.00
|
0
|
0.00
|
2
|
2.70
|
1
|
2.44
|
0
|
0.00
|
0
|
0.00
|
0
|
0.00
|
0.1932
|
|
Steptococcus sp. + Candida sp.
|
0
|
0.00
|
0
|
0.00
|
0
|
0.00
|
0
|
0.00
|
0
|
0.00
|
0
|
0.00
|
1
|
4.00
|
0.4232
|
4.5 Repartition of microorganism according to
theirsusceptibility to the class of antimicrobial drugs
The repartition of microorganism according to their
susceptibility to classes of antimicrobial drugsshows that C. albicans
was more sensible both in azole (75.00%) and polyenes (58.33%). E. coli was
more sensitive to Macrolides (78.21%) whereas it was more resistant to
cephalosporine (55.13%), penicillin (84.62%), Fluoroquinolones (68.57%) and
Carbapenem (58.97%).
Table 6: Repartition of microorganism according to
theirsusceptibility to classes of antimicrobial drugs
|
|
Macrolides
|
Cephalosporin
|
Penicillin
|
Azole
|
polyenes
|
Fluoroquinolones
|
Carbapenem
|
|
|
|
|
|
|
|
|
|
|
|
Microorganisms
|
Sensitive
(%)
|
Resistant
(%)
|
Sensitive
(%)
|
Resistant
(%)
|
Sensitive
(%)
|
Resistant
(%)
|
Sensitive
(%)
|
Resistant
(%)
|
Sensitive
(%)
|
Resistant
(%)
|
Sensitive
(%)
|
Resistant
(%)
|
Sensitive
(%)
|
Resistant
(%)
|
TOTAL
(%)
|
|
C. albicans
|
NA
|
NA
|
NA
|
NA
|
NA
|
NA
|
75.00
|
25.00
|
58.33
|
41.67
|
NA
|
NA
|
NA
|
NA
|
12
|
|
E. coli
|
78.21
|
21.79
|
44.87
|
55.13
|
15.38
|
84.62
|
NA
|
NA
|
NA
|
NA
|
41.03
|
58.97
|
41.03
|
58.97
|
78
|
|
Klebsiella sp.
|
77.14
|
22.86
|
48.57
|
51.43
|
17.14
|
82.86
|
NA
|
NA
|
NA
|
NA
|
31.43
|
68.57
|
31.43
|
68.57
|
35
|
|
Proteus sp.
|
69.23
|
30.77
|
26.92
|
73.08
|
19.23
|
80.77
|
NA
|
NA
|
NA
|
NA
|
42.31
|
57.69
|
30.77
|
69.23
|
26
|
|
P. stuartii
|
90.91
|
9.09
|
63.64
|
36.36
|
27.27
|
72.73
|
NA
|
NA
|
NA
|
NA
|
27.27
|
72.73
|
63.64
|
36.36
|
11
|
|
P. aeruginosa
|
100.00
|
0.00
|
33.33
|
66.67
|
66.67
|
33.33
|
NA
|
NA
|
NA
|
NA
|
33.33
|
66.67
|
66.67
|
33.33
|
3
|
|
Serratia sp.
|
100.00
|
0.00
|
0.00
|
100.00
|
0.00
|
100.00
|
NA
|
NA
|
NA
|
NA
|
0.00
|
100.00
|
0.00
|
100.00
|
1
|
|
Staphylococcus sp.
|
77.61
|
22.39
|
41.79
|
58.21
|
35.82
|
64.18
|
NA
|
NA
|
NA
|
NA
|
1.49
|
98.51
|
35.82
|
64.18
|
67
|
|
Steptococcus sp.
|
50.00
|
50.00
|
0.00
|
100.00
|
50.00
|
50.00
|
NA
|
NA
|
NA
|
NA
|
0.00
|
100.00
|
50.00
|
50.00
|
2
|
|
E. coli + Candida sp.
|
66.67
|
33.33
|
33.33
|
66.67
|
16.67
|
83.33
|
66.67
|
33.33
|
83.33
|
16.67
|
50.00
|
50.00
|
0.00
|
100.00
|
6
|
|
Klebsiella sp. + Candida sp.
|
66.67
|
33.33
|
33.33
|
66.67
|
0.00
|
100.00
|
0.00
|
100.00
|
66.67
|
33.33
|
33.33
|
66.67
|
66.67
|
33.33
|
3
|
|
Staphylococcus sp. + Candida sp.
|
66.67
|
33.33
|
100.00
|
0.00
|
33.33
|
66.67
|
66.67
|
33.33
|
0.00
|
100.00
|
33.33
|
66.67
|
33.33
|
66.67
|
3
|
|
Steptococcus sp. + Candida sp.
|
100.00
|
0.00
|
0.00
|
100.00
|
0.00
|
100.00
|
100.00
|
0.00
|
0.00
|
100.00
|
0.00
|
100.00
|
0.00
|
100.00
|
1
|
4.6 Comparison of the susceptibility of antimicrobial
drugs to microorganism according to sex
No significant difference was observe on susceptibility of
microorganism according to sex except with Streptococcus where significant
difference were observe with penicillin and Fluroquinolonesand with these, the
resistance was high in females.
Table 7;Comparison of the susceptibility of
microorganisms to classes of antimicrobial drugs according to sex
|
|
Macrolides
|
|
Cephalosporin
|
Penicillin
|
Azole
|
polyenes
|
Fluoroquinolones
|
Carbapenem
|
|
Microorganisms
|
Sensitive
|
Resistant
|
Sensitive
|
Resistant
|
Sensitive
|
Resistant
|
Sensitive
|
Resistant
|
Sensitive
|
Resistant
|
Sensitive
|
Resistant
|
Sensitive
|
Resistant
|
|
C. albicans
|
NA
|
NA
|
NA
|
NA
|
NA
|
NA
|
9
|
3
|
7
|
5
|
NA
|
NA
|
NA
|
NA
|
|
Female
|
NA
|
NA
|
NA
|
NA
|
NA
|
NA
|
9
|
3
|
7
|
5
|
NA
|
NA
|
NA
|
NA
|
|
Male
|
NA
|
NA
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
|
P-value
|
NA
|
NA
|
NA
|
>0,9999
|
>0,9999
|
NA
|
NA
|
|
|
E. coli
|
61
|
17
|
35
|
43
|
12
|
66
|
NA
|
NA
|
NA
|
NA
|
32
|
46
|
32
|
46
|
|
Female
|
53
|
14
|
28
|
39
|
8
|
59
|
NA
|
NA
|
NA
|
NA
|
27
|
40
|
30
|
37
|
|
Male
|
8
|
3
|
7
|
4
|
4
|
7
|
NA
|
NA
|
NA
|
NA
|
5
|
6
|
2
|
9
|
|
P-value
|
0.6968
|
0.2058
|
0.0599
|
NA
|
NA
|
0.7526
|
0.1134
|
|
|
Klebsiella sp.
|
27
|
8
|
17
|
18
|
6
|
29
|
NA
|
NA
|
NA
|
NA
|
11
|
24
|
11
|
24
|
|
Female
|
13
|
5
|
8
|
10
|
2
|
16
|
NA
|
NA
|
NA
|
NA
|
6
|
12
|
6
|
12
|
|
Male
|
14
|
3
|
9
|
8
|
4
|
13
|
NA
|
NA
|
NA
|
NA
|
5
|
12
|
5
|
12
|
|
P-value
|
0.6906
|
0.7395
|
0.4018
|
NA
|
NA
|
>0,9999
|
>0,9999
|
|
|
Proteus sp.
|
18
|
8
|
7
|
19
|
5
|
21
|
NA
|
NA
|
NA
|
NA
|
11
|
15
|
8
|
18
|
|
Female
|
15
|
4
|
6
|
13
|
4
|
15
|
NA
|
NA
|
NA
|
NA
|
9
|
10
|
6
|
13
|
|
Male
|
3
|
4
|
1
|
6
|
1
|
6
|
NA
|
NA
|
NA
|
NA
|
2
|
5
|
2
|
5
|
|
P-value
|
0.149
|
0.6288
|
>0,9999
|
NA
|
NA
|
0.6576
|
>0,9999
|
|
|
P. stuartii
|
10
|
1
|
7
|
4
|
3
|
8
|
NA
|
NA
|
NA
|
NA
|
3
|
8
|
7
|
4
|
|
Female
|
9
|
1
|
6
|
4
|
3
|
7
|
NA
|
NA
|
NA
|
NA
|
3
|
7
|
6
|
4
|
|
Male
|
1
|
0
|
1
|
0
|
0
|
1
|
NA
|
NA
|
NA
|
NA
|
0
|
1
|
1
|
0
|
|
P-value
|
>0,9999
|
>0,9999
|
>0,9999
|
NA
|
NA
|
>0,9999
|
>0,9999
|
|
|
P. aeruginosa
|
3
|
0
|
1
|
2
|
2
|
1
|
NA
|
NA
|
NA
|
NA
|
1
|
2
|
2
|
1
|
|
Female
|
3
|
0
|
1
|
2
|
3
|
0
|
NA
|
NA
|
NA
|
NA
|
1
|
2
|
2
|
1
|
|
Male
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
|
P-value
|
>0,9999
|
>0,9999
|
>0,9999
|
NA
|
NA
|
>0,9999
|
>0,9999
|
|
|
Serratia sp.
|
1
|
0
|
0
|
1
|
0
|
1
|
NA
|
NA
|
NA
|
NA
|
0
|
1
|
0
|
1
|
|
Female
|
1
|
0
|
0
|
1
|
0
|
1
|
NA
|
NA
|
NA
|
NA
|
0
|
1
|
0
|
1
|
|
Male
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
|
P-value
|
>0,9999
|
>0,9999
|
>0,9999
|
NA
|
NA
|
>0,9999
|
>0,9999
|
|
|
Staphylococcus sp.
|
52
|
15
|
28
|
39
|
24
|
43
|
NA
|
NA
|
NA
|
NA
|
22
|
45
|
24
|
43
|
|
Female
|
40
|
12
|
20
|
32
|
22
|
30
|
NA
|
NA
|
NA
|
NA
|
12
|
40
|
18
|
34
|
|
Male
|
12
|
3
|
8
|
7
|
1
|
14
|
NA
|
NA
|
NA
|
NA
|
10
|
5
|
6
|
9
|
|
P-value
|
>0,9999
|
0.3777
|
0.0125
|
NA
|
NA
|
0.0036
|
0.7643
|
|
|
Steptococcus sp.
|
1
|
1
|
0
|
2
|
1
|
1
|
NA
|
NA
|
NA
|
NA
|
0
|
2
|
1
|
1
|
|
Female
|
1
|
1
|
0
|
2
|
1
|
1
|
NA
|
NA
|
NA
|
NA
|
0
|
2
|
1
|
1
|
|
Male
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
|
P-value
|
>0,9999
|
>0,9999
|
>0,9999
|
NA
|
NA
|
>0,9999
|
>0,9999
|
|
|
E. coli + Candida sp.
|
4
|
2
|
2
|
4
|
1
|
5
|
4
|
2
|
5
|
1
|
3
|
3
|
0
|
6
|
|
Female
|
4
|
1
|
1
|
4
|
1
|
4
|
3
|
2
|
4
|
1
|
3
|
2
|
0
|
5
|
|
Male
|
0
|
1
|
1
|
0
|
0
|
1
|
1
|
0
|
1
|
0
|
0
|
1
|
0
|
1
|
|
P-value
|
0.3333
|
0.3333
|
>0,9999
|
>0,9999
|
>0,9999
|
>0,9999
|
|
|
|
Klebsiella sp. + Candida sp.
|
2
|
1
|
1
|
2
|
0
|
3
|
0
|
3
|
2
|
1
|
1
|
2
|
2
|
1
|
|
Female
|
2
|
1
|
1
|
2
|
0
|
3
|
0
|
3
|
2
|
1
|
1
|
2
|
2
|
1
|
|
Male
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
P-value
|
>0,9999
|
>0,9999
|
>0,9999
|
>0,9999
|
>0,9999
|
>0,9999
|
>0,9999
|
|
|
Staphylococcus sp. + Candida sp.
|
2
|
1
|
3
|
0
|
1
|
2
|
2
|
1
|
0
|
3
|
1
|
2
|
1
|
2
|
|
Female
|
2
|
1
|
3
|
0
|
1
|
2
|
2
|
1
|
0
|
3
|
1
|
2
|
1
|
2
|
|
Male
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
P-value
|
>0,9999
|
|
>0,9999
|
>0,9999
|
|
>0,9999
|
>0,9999
|
|
|
Steptococcus sp. + Candida sp.
|
1
|
0
|
0
|
1
|
0
|
1
|
1
|
0
|
0
|
1
|
0
|
1
|
0
|
1
|
|
Female
|
1
|
0
|
0
|
1
|
0
|
1
|
1
|
0
|
0
|
1
|
0
|
1
|
0
|
1
|
|
Male
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
P-value
|
>0,9999
|
>0,9999
|
>0,9999
|
>0,9999
|
>0,9999
|
>0,9999
|
>0,9999
|
|
4.7; Comparison of the susceptibility of microorganisms
to antimicrobial drugs according to age range
Apart fromC. albicans where significant difference was
observe in Azoles with (P=0.0273), no significant difference was observe with
other microorganism according to age range.
Table 8; Comparison of the susceptibility of
microorganism to antimicrobial drugs according to age range
|
Microorganisms
|
Macrolides
|
Cephalosporin
|
Penicillin
|
Azole
|
polyenes
|
Fluoroquinolones
|
Carbapenem
|
|
|
Sensitive
|
Resistant
|
Sensitive
|
Resistant
|
Sensitive
|
Resistant
|
Sensitive
|
Resistant
|
Sensitive
|
Resistant
|
Sensitive
|
Resistant
|
Sensitive
|
Resistant
|
TOTAL
|
|
C. albicans
|
NA
|
NA
|
NA
|
NA
|
NA
|
NA
|
9
|
3
|
7
|
5
|
NA
|
NA
|
NA
|
NA
|
12
|
|
[1-10]
|
NA
|
NA
|
NA
|
NA
|
NA
|
NA
|
1
|
2
|
2
|
1
|
NA
|
NA
|
NA
|
NA
|
3
|
|
[11-20]
|
NA
|
NA
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
|
[21-30]
|
NA
|
NA
|
NA
|
NA
|
NA
|
NA
|
5
|
0
|
3
|
2
|
NA
|
NA
|
NA
|
NA
|
5
|
|
[31-40]
|
NA
|
NA
|
NA
|
NA
|
NA
|
NA
|
4
|
0
|
2
|
2
|
NA
|
NA
|
NA
|
NA
|
4
|
|
[41-50]
|
NA
|
NA
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
|
[51-60]
|
NA
|
NA
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
|
[61-70]
|
NA
|
NA
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
|
[71-80]
|
NA
|
NA
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
|
P-value
|
NA
|
NA
|
NA
|
0.0273
|
0.9767
|
NA
|
NA
|
|
|
E. coli
|
61
|
17
|
35
|
43
|
12
|
66
|
NA
|
NA
|
NA
|
NA
|
32
|
46
|
32
|
46
|
78
|
|
[1-10]
|
16
|
4
|
9
|
11
|
3
|
17
|
NA
|
NA
|
NA
|
NA
|
3
|
17
|
10
|
10
|
20
|
|
[11-20]
|
3
|
1
|
1
|
3
|
1
|
3
|
NA
|
NA
|
NA
|
NA
|
2
|
2
|
1
|
3
|
4
|
|
[21-30]
|
12
|
2
|
4
|
10
|
1
|
13
|
NA
|
NA
|
NA
|
NA
|
8
|
6
|
4
|
10
|
14
|
|
[31-40]
|
8
|
3
|
5
|
6
|
1
|
10
|
NA
|
NA
|
NA
|
NA
|
7
|
4
|
3
|
8
|
11
|
|
[41-50]
|
9
|
4
|
8
|
5
|
4
|
9
|
NA
|
NA
|
NA
|
NA
|
5
|
8
|
8
|
5
|
13
|
|
[51-60]
|
5
|
2
|
4
|
3
|
1
|
6
|
NA
|
NA
|
NA
|
NA
|
2
|
5
|
1
|
6
|
7
|
|
[61-70]
|
8
|
1
|
4
|
5
|
1
|
8
|
NA
|
NA
|
NA
|
NA
|
5
|
4
|
5
|
4
|
9
|
|
[71-80]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
P-value
|
0.9526
|
0.6725
|
0.6972
|
NA
|
NA
|
0.0938
|
0.2408
|
|
|
Klebsiella sp.
|
27
|
8
|
17
|
18
|
11
|
24
|
NA
|
NA
|
NA
|
NA
|
11
|
24
|
11
|
24
|
35
|
|
[1-10]
|
6
|
0
|
2
|
4
|
3
|
3
|
NA
|
NA
|
NA
|
NA
|
2
|
4
|
4
|
2
|
6
|
|
[11-20]
|
5
|
3
|
6
|
2
|
2
|
6
|
NA
|
NA
|
NA
|
NA
|
3
|
5
|
2
|
6
|
8
|
|
[21-30]
|
7
|
2
|
3
|
6
|
5
|
4
|
NA
|
NA
|
NA
|
NA
|
3
|
6
|
3
|
6
|
9
|
|
[31-40]
|
1
|
1
|
1
|
1
|
0
|
2
|
NA
|
NA
|
NA
|
NA
|
1
|
1
|
1
|
1
|
2
|
|
[41-50]
|
3
|
0
|
1
|
2
|
0
|
3
|
NA
|
NA
|
NA
|
NA
|
0
|
3
|
1
|
2
|
3
|
|
[51-60]
|
5
|
2
|
4
|
3
|
1
|
6
|
NA
|
NA
|
NA
|
NA
|
2
|
5
|
1
|
6
|
7
|
|
[61-70]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
[71-80]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
P-value
|
0.4657
|
0.5326
|
0.2367
|
NA
|
NA
|
0.865
|
0.4712
|
|
|
Proteus sp.
|
18
|
8
|
7
|
19
|
5
|
21
|
NA
|
NA
|
NA
|
NA
|
11
|
15
|
8
|
18
|
26
|
|
[1-10]
|
3
|
0
|
1
|
2
|
0
|
3
|
NA
|
NA
|
NA
|
NA
|
0
|
3
|
3
|
0
|
3
|
|
[11-20]
|
2
|
0
|
0
|
2
|
0
|
2
|
NA
|
NA
|
NA
|
NA
|
2
|
0
|
1
|
1
|
2
|
|
[21-30]
|
7
|
4
|
3
|
8
|
4
|
7
|
NA
|
NA
|
NA
|
NA
|
5
|
6
|
2
|
9
|
11
|
|
[31-40]
|
5
|
0
|
3
|
2
|
0
|
5
|
NA
|
NA
|
NA
|
NA
|
2
|
3
|
1
|
4
|
5
|
|
[41-50]
|
0
|
1
|
0
|
1
|
0
|
1
|
NA
|
NA
|
NA
|
NA
|
1
|
0
|
0
|
1
|
1
|
|
[51-60]
|
1
|
1
|
0
|
2
|
0
|
2
|
NA
|
NA
|
NA
|
NA
|
1
|
1
|
0
|
2
|
2
|
|
[61-70]
|
0
|
2
|
0
|
2
|
1
|
1
|
NA
|
NA
|
NA
|
NA
|
0
|
2
|
1
|
1
|
2
|
|
[71-80]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
P-value
|
0.0689
|
0.4908
|
0.4947
|
NA
|
NA
|
0.2484
|
0.1303
|
|
|
P. stuartii
|
10
|
1
|
7
|
4
|
3
|
8
|
NA
|
NA
|
NA
|
NA
|
3
|
8
|
7
|
4
|
11
|
|
[1-10]
|
4
|
1
|
3
|
2
|
0
|
5
|
NA
|
NA
|
NA
|
NA
|
0
|
5
|
4
|
1
|
5
|
|
[11-20]
|
2
|
0
|
1
|
1
|
1
|
1
|
NA
|
NA
|
NA
|
NA
|
1
|
1
|
2
|
0
|
2
|
|
[21-30]
|
1
|
0
|
0
|
1
|
0
|
1
|
NA
|
NA
|
NA
|
NA
|
1
|
0
|
0
|
1
|
1
|
|
[31-40]
|
2
|
0
|
2
|
0
|
1
|
1
|
NA
|
NA
|
NA
|
NA
|
1
|
1
|
1
|
1
|
2
|
|
[41-50]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
[51-60]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
[61-70]
|
1
|
0
|
1
|
0
|
1
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
1
|
0
|
1
|
1
|
|
[71-80]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
P-value
|
|
|
|
NA
|
NA
|
0.1634
|
0.1983
|
|
|
P. aeruginosa
|
3
|
0
|
1
|
2
|
2
|
1
|
NA
|
NA
|
NA
|
NA
|
0
|
3
|
2
|
1
|
3
|
|
[1-10]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
[11-20]
|
1
|
0
|
0
|
1
|
0
|
1
|
NA
|
NA
|
NA
|
NA
|
0
|
1
|
0
|
1
|
1
|
|
[21-30]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
[31-40]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
[41-50]
|
1
|
0
|
0
|
1
|
1
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
1
|
1
|
0
|
1
|
|
[51-60]
|
1
|
0
|
1
|
0
|
1
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
1
|
1
|
0
|
1
|
|
[61-70]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
|
|
[71-80]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
P-value
|
|
|
|
NA
|
NA
|
NA
|
>0.9999
|
|
|
Serratia sp.
|
1
|
0
|
0
|
1
|
0
|
1
|
NA
|
NA
|
NA
|
NA
|
0
|
1
|
0
|
1
|
1
|
|
[1-10]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
[11-20]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
[21-30]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
[31-40]
|
1
|
0
|
0
|
1
|
0
|
1
|
NA
|
NA
|
NA
|
NA
|
0
|
1
|
0
|
1
|
1
|
|
[41-50]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
[51-60]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
[61-70]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
[71-80]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
P-value
|
|
|
|
NA
|
NA
|
>0.9999
|
>0.9999
|
|
|
Staphylococcus sp.
|
52
|
15
|
28
|
39
|
24
|
43
|
NA
|
NA
|
NA
|
NA
|
17
|
50
|
24
|
43
|
67
|
|
[1-10]
|
3
|
4
|
3
|
4
|
2
|
5
|
NA
|
NA
|
NA
|
NA
|
2
|
5
|
2
|
5
|
7
|
|
[11-20]
|
4
|
2
|
4
|
2
|
3
|
3
|
NA
|
NA
|
NA
|
NA
|
2
|
4
|
2
|
4
|
6
|
|
[21-30]
|
25
|
6
|
12
|
19
|
13
|
18
|
NA
|
NA
|
NA
|
NA
|
8
|
23
|
16
|
15
|
31
|
|
[31-40]
|
10
|
1
|
3
|
8
|
4
|
7
|
NA
|
NA
|
NA
|
NA
|
2
|
9
|
1
|
10
|
11
|
|
[41-50]
|
5
|
0
|
2
|
3
|
2
|
3
|
NA
|
NA
|
NA
|
NA
|
1
|
4
|
1
|
4
|
5
|
|
[51-60]
|
2
|
1
|
1
|
2
|
0
|
3
|
NA
|
NA
|
NA
|
NA
|
2
|
1
|
0
|
3
|
3
|
|
[61-70]
|
2
|
1
|
2
|
1
|
0
|
3
|
NA
|
NA
|
NA
|
NA
|
0
|
3
|
1
|
2
|
3
|
|
[71-80]
|
1
|
0
|
1
|
0
|
0
|
1
|
NA
|
NA
|
NA
|
NA
|
0
|
1
|
1
|
0
|
1
|
|
P-value
|
|
|
|
NA
|
NA
|
0.5855
|
0.1397
|
|
|
Steptococcus sp.
|
1
|
1
|
0
|
2
|
1
|
1
|
NA
|
NA
|
NA
|
NA
|
0
|
2
|
1
|
1
|
2
|
|
[1-10]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
[11-20]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
[21-30]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
[31-40]
|
0
|
1
|
0
|
1
|
1
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
1
|
0
|
1
|
1
|
|
[41-50]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
[51-60]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
[61-70]
|
1
|
0
|
0
|
1
|
0
|
1
|
NA
|
NA
|
NA
|
NA
|
0
|
1
|
1
|
0
|
1
|
|
[71-80]
|
0
|
0
|
0
|
0
|
0
|
0
|
NA
|
NA
|
NA
|
NA
|
0
|
0
|
0
|
0
|
0
|
|
P-value
|
7
|
NA
|
NA
|
NA
|
NA
|
>0.9999
|
>0.9999
|
|
|
E. coli + Candida sp.
|
4
|
2
|
2
|
4
|
1
|
5
|
4
|
2
|
5
|
1
|
3
|
3
|
0
|
6
|
6
|
|
[1-10]
|
1
|
1
|
1
|
1
|
0
|
2
|
1
|
1
|
2
|
0
|
0
|
2
|
0
|
2
|
2
|
|
[11-20]
|
0
|
1
|
1
|
0
|
1
|
0
|
1
|
0
|
1
|
0
|
0
|
1
|
0
|
1
|
1
|
|
[21-30]
|
1
|
0
|
0
|
1
|
0
|
1
|
1
|
0
|
1
|
0
|
1
|
0
|
0
|
1
|
1
|
|
[31-40]
|
2
|
0
|
0
|
2
|
0
|
2
|
1
|
1
|
1
|
1
|
2
|
0
|
0
|
2
|
2
|
|
[41-50]
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
[51-60]
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
[61-70]
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
[71-80]
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
P-value
|
0.2898
|
0.2898
|
0.1116
|
0.8266
|
0.4936
|
0.1116
|
>0.9999
|
|
|
Klebsiella sp. + Candida sp.
|
2
|
0
|
1
|
1
|
0
|
2
|
0
|
2
|
2
|
0
|
1
|
1
|
2
|
0
|
2
|
|
[1-10]
|
1
|
0
|
0
|
1
|
0
|
1
|
0
|
1
|
0
|
1
|
1
|
0
|
0
|
1
|
1
|
|
[11-20]
|
1
|
0
|
0
|
1
|
0
|
1
|
0
|
1
|
1
|
0
|
0
|
1
|
1
|
0
|
1
|
|
[21-30]
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
[31-40]
|
0
|
1
|
1
|
0
|
0
|
1
|
0
|
1
|
1
|
0
|
0
|
1
|
1
|
0
|
1
|
|
[41-50]
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
[51-60]
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
[61-70]
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
[71-80]
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
P-value
|
0.3865
|
0.6592
|
NA
|
NA
|
0.2231
|
0.2231
|
0.2231
|
|
|
Staphylococcus sp. + Candida sp.
|
2
|
1
|
3
|
0
|
1
|
2
|
2
|
1
|
0
|
3
|
1
|
2
|
1
|
2
|
3
|
|
[1-10]
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
[11-20]
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
[21-30]
|
1
|
1
|
2
|
0
|
0
|
2
|
1
|
1
|
0
|
2
|
0
|
2
|
1
|
1
|
2
|
|
[31-40]
|
1
|
0
|
1
|
0
|
1
|
0
|
1
|
0
|
0
|
1
|
1
|
0
|
0
|
1
|
1
|
|
[41-50]
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
[51-60]
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
[61-70]
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
[71-80]
|
0
|
0
|
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
P-value
|
0.3865
|
NA
|
0.0833
|
0.3865
|
NA
|
0.0833
|
0.3865
|
|
|
Steptococcus sp. + Candida sp.
|
1
|
0
|
0
|
1
|
0
|
1
|
1
|
0
|
0
|
1
|
0
|
1
|
0
|
1
|
1
|
|
[1-10]
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
[11-20]
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
[21-30]
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
[31-40]
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
[41-50]
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
[51-60]
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
[61-70]
|
1
|
0
|
0
|
1
|
0
|
1
|
1
|
0
|
0
|
1
|
0
|
1
|
0
|
1
|
1
|
|
[71-80]
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
P-value
|
NA
|
NA
|
NA
|
NA
|
NA
|
NA
|
NA
|
|
5.1 DISCUSSION
Studies have demonstrated a
geographicvariation in etiologic characteristics of UTI and their resistance
patterns to antimicrobial drugs. Therefore to successfully eradicate UTI by
treatment, knowledge of local etiologic agents and their antimicrobial drugs
susceptibility is of great value (Nzalie et al 2016).
Out 248 samples of the isolates of UTI collected94.75%
(235/248) showed single growth and 5.25% (13/248) showed mix or multiple
growth. This may be justify by the fact that a typical infection may result
from a single pathogen or the association of more than one pathogen, as well as
aco-infection arising from poor or no treatment of a typical infection.Nine
strains of uropathogens were isolated, of which 8 were bacteria and 1 fungus.
This may be justify by the fact that bacteria are found in the bowel and live
as normal flora and often result from fecal and perianal areas as such easily
invade the tissues of the urinary tract. This result corroborate with those
obtain by Akoachere et al. (2012). In this study, the rate of
positivity of UTI in females was higher than in males. Thishigh prevalence
among females is due to the nature of their urinogental tract; the urethra of
the female is much shorter and closer to the anus than in males .Such result
have beenreportedby Oyaert (Oyaert et al., 2018). Furthermore, gram
positive isolates in this study accounts for 29.44% (27.83% for single growth
and 1.61% for mix growth) while gram negative accounts for 65.72% (62.09% for
single growth and 3.63% for mix growth) with the most common gram negative
isolates beenE coli (31.45%) and the most common gram positive isolate
been Staphylococcus sp (27.02%).Such result results have been reported
in two towns in Cameroon by Akoachere et al. (2012).The fungus(Candida
albicans) account for 4.84% of the uropathogens this low prevalence of fungi
infection may be due lack of identification process in our study area. This
result is differ from what obtain by Rahimi et al. (Rahimiet
al., 2018). In addition, a variation in distribution of etiologic agents
with age was observed in this study with age range [21-30] been the most
infected and the least infected was the age range [71-80] this high prevalence
found in the age range from [21-30] is due to the fact that it is this age that
people are more sexually active in our environment and most of them are not
using preventive materials and this sexuality decrease with age and occupation.
Susceptibility pattern wasanalyzed according to classes of
antimicrobial drugs, we observe that, no matter the class of antimicrobial
drugs and no matter the susceptibility this was high in female compare to male
and the most susceptible microorganism was E. Coli with macrolide
whereas Proteus sp found to be more resistant to all the class of antimicrobial
drugs. This high resistance though not been significant among female may be due
to multiple infection thatlead to non-specificity of drugs prescribe but it may
also due to incomplete medication that is once the treatment is prescribe, the
take it well but once they feel a little bit good the abandon the remaining
treatment. This result corroborate with the study carried out in Kenya
byGachuhi. (2017)
Availability and indiscriminate use of commonly used
antibioticswithout health care workers prescription (self-medication) lead to
an increased multidrug resistance. Due to the increasing multidrug resistance
among uropathogens, the health care workers are left with a limited choice of
routinely used antibiotics to choose from for the treatment of urinary tract
infections. It is seen in this study where Nitrofurantoin which is mostly used
as the drug of choice for treatment was not present among the antimicrobial
drugs .This can be attributed the fact that bacteria undergo mutation which
makes their susceptibility vary from one geographical area to the other
(Jaiswal et al., 2013).
5.2-Conclusion
This study reveals a familiar pattern with respect to the
species of uropathogens involved in UTIs with the principal cause being the
gram-negative bacteria, E. coli and Klebsiella spas the
leading ones. However gram positive were also among the etiology with
Staphylococcus sp being the leading one .This study alsoshowed
considerable bacterial resistance to common empirically prescribed
antibiotics.The worldwide trend of empirically treating UTI may not apply to
specific geographical regions. This is as a result of the etiological variation
from one region to another.Macrolides still has a very high sensitivity level
against P. aeruginosa and Serratia Sp (100%) as well as to other etiologic
agents. As such this study suggests that macrolides are better for the
empirical treatment of UTIs.
5.3-Limitations of the study
A limitation to this study is the inability to sort out the
major risk factors of UTIs and the leading cause of antimicrobial drugs
resistance among the patients attending the DHN given that it's a retrospective
study and there is no interaction with the patients. Also due to the large
number of isolates obtained from this study, we were unable to characterize the
Gram positive isolates and some gram negative as well to permit their
identification to species level.
5.4-Recommendations
· A continuous surveillance of etiological profile and
antimicrobial drugs to the currently used antibiotics in management of urinary
tract infections in the whole country Cameroon.
· Enforcement of policies to be followed in pharmacy
and by medical doctors like to prevent misuse of antimicrobial drugs by giving
prescription to only patients with results of culture and sensitivity and
therefore treatment of UTIs should be based on the etiology and sensitivity in
order to limit multidrug resistance.
· Health care workers should enforce health education to
patients in order to create awareness of the risk factors and how to avoid them
as well as to adhere to the treatment therebyreducing drug resistance.
· Destruction of free markets.
· The need to establish local and national antimicrobial
resistance monitoring systems in Cameroon to provide information for the
development of treatment guidelines.
Akoachera J-FTK, Suylika Y, Akum N, Seraphine EN
(2012).Etiologic profile and antimicrobial susceptibility of
community-acquired urinary tract infection in two Cameroonian towns BMC
Research Notes, 5:219
Ana L. Flores-Mireles, Jennifer N. Walker, Michael
Caparon, and Scott J. Hultgren.(2015) Urinary tract infections:
epidemiology, mechanisms of infection and treatment options. HHS Public
Access; 1-34
Anuli S. John, Clement I. Mboto and Basseye Agbo
(2016). A review on the prevalence and predisposing factors
responsible for urinary tract infection among adults. EuropeanJournal of
Experimental Biology:7-11.
Christine M. Chu, MD; Jerry L. Lowder, MD. (2018)
Diagnosis and treatment of urinary tract infections across age
groups. American Journal of Obstetrics & Gynecology;1-12.
Dorgelesse Francine Kouemo Motse, Guy Pascal Ngaba,
Loick Pradel Kojom, Danielle Christiane Kedy Koum, Cecile Ebongue Okalla,
Calixte Ida Penda, Evelyn Mah5, Andreas Chiabi5, Dieudonné
Désiré Adiogo. (2019)Predictors of Urinary
Tract Infection and Their Diagnostic Performances among Cameroonian
Under-five.Journal of Microbiology and Infectious Diseases ;1-10.
Florian Wagenlehner , Zafer Tandogdu , Riccardo
Bartoletti, Tommaso Cai ,Mete Cek Ekaterina Kulchavenya , Béla
Köves, Kurt Naber, Tamara Perepanova , Peter Tenke ,Björn Wullt ,
Florian Bogenhard and Truls Erik Bjerklund Johansen. (2016)
The global Prevalence of Infections in Urology Study: A Long Term Worldwide
Surveillance Study on Urological Infections.
www.mdpi.com/journal/pathogens
Foxman B (2010). The epidemiology of urinary
tract infection. Nature Reviews Urology, vol. 7;653-660
Griebling TL. (2005) .Urologic diseases in
America project: Trends in resource use for urinary tract infections in men
PubMed ;1288-1294
Hooton,T. M., Bradley,S. F. and Cardenas.D (
2010)Diagnosis, prevention and treatment of catheter-associated
urinary tract infection in adults: Infectious diseases society of America.;
625-663
Ke He, Yun Hu, Jun-Cheng Shi, Yun-Qing Zhu, Xiao-Ming
Mao. (2018)Prevalence, risk factors and microorganisms of
urinary tract infections in patients with type 2 diabetes mellitus: a
retrospective study in China. Therapeutics and Clinical Risks
management, 14: 403-408
Loveline M Ndamason, Wiliane JT Marbou, Victor Kuete.
(2019)Urinary tract infections, bacterial resistance and immunological
status: a cross sectional study in pregnant and non-pregnant women at Mbouda
Ad-Lucem Hospital. African Health Sciences, 19: 1-11
Martha Medina and Edgardo Castillo-Pino.
(2019).An introduction to the epidemiology and burden of urinary tract
infections. journals.sagepub.com/home/tau; 1-5
Mehta, M. Bhardwaj, S. and Sharma, J. (2013).
Screening of urinary isolates for the prevalence and antimicrobial
susceptibility of enterobacteria other than E. coli.
Michael Davenport, Kathleen E. Mach, Linda M. Dairiki
Shortliffe, Niaz Banaei, Tza-Huei Wang, and Joseph C. Liao
(2017).New and developing diagnostic technologies for urinary
tract infections.HHS Public Access 1-33
Monica Cheesbrough. (2006) District
laboratory practice in tropical countries, second edition update.Cambridge
low price edition part 1
Nicolle, L.E(2008) Uncomplicated
urinary tract infection in adults including uncomplicated pyelonephritis.
Pubmed
Nzalie Rolf Nyah-tuku, Hortense Kamga Gonsu and
Sinata Koulla-Shiro (2016)Bacterial Etiology and Antibiotic
Resistance Profile of Community-Acquired Urinary Tract Infections in a
Cameroonian City. Hindawi Publishing Corporation International Journal of
Microbiology;1- 6
Odoki Martin, Adamu Almustapha Aliero , Julius
Tibyangye ,Josephat Nyabayo Maniga,Eddie Wampande, Charles Drago Kato, Ezera
Agwu and Joel Bazira (2019).Prevalence of Bacterial Urinary Tract
Infections and Associated Factors among Patients Attending Hospitals in
Bushenyi District, Uganda. Hindawi International Journal
of Microbiology; 1-8.
Oluwafemi TT, Akinbodewa AA, Ogunleye A, Adejumo OA.
(2018) Urinary tract infections and antibiotic sensitivity pattern of
uropathogens in a tertiary hospital in South West, Nigeria.Sahel medical
journal 18-22
Otajevwo FD, Amedu SS.(2015) Community
acquired urinary tract infection prevalence in a tertiary institution based in
Evbuobanosa, Edo State, Nigeria.Docplayer
Oyaert Matthijs, Britt Van Meensel, Reinoud
Cartuyvels, Johan Frans, Wim Laffut,Patricia Vandecandelaere, Hans De
Beenhouwer (2018). Laboratory diagnosis of urinary tract infections:
Towards a BILULU consensus guideline, Journal of Microbiological Methods:
92-99
Rahem Khoshbakht, Ayub Salimi, Hessamaddin Shirzad
Aski, Hale Keshavarzi.(2013) Antibiotics susceptibility of bacterial
strains isolated from UTIs in Karaj Iran. Jundishapur Journal of
Microbiology 86-90.
Rahimi A, Saragih R H* and Nainggolan R (2018).
Antimicrobial resistance profile of urinary tract infection at a
secondary care hospital in Medan, Indonesia. Conf. Series:
Earth and Environmental Science125: 1-5
Rezina Parveen, Md. Abdullah Yusuf, Ishrat Sharmin,
Md. Saiful Islam,Ina Rahim.(2015) Antibiotic Sensitivity of Bacteria
Causing Urinary Tract Infection. Bangladesh Journal of Infectious Diseases
Volume 2.
Singh Randhir .k, Dewasy Bijoylakshmi, Mallick Ram .L
and Kafle Tara .K.(2016)Prevalence of antibiotics sensitivity pattern
of uropathogens in patients of different age-groups from western region of
Nepal. International journal of medical research and health science 1-7
Tibi Gachuhi George.
(2017)Antibiotic Susceptibility Pattern Of Bacterial Uropathogens
Isolated From Patients In Nakuru Level 5 Hospital, Kenya
Valerie phillips. (2013)Guidelines for the
Prevention and Treatment of Urinary Tract Infections (UTIs) in Continuing Care
Settings. Saskatchewan Infection Prevention and Control Program; 1-28.
Wubalem Desta Seifu and Alemayehu Desalegn Gebissa
(2017) Prevalence and antibiotics susceptibility of uropathogens from
cases of urinary tract infections in sashemene referral hospital in
Ethiopia. BMC Infectious Diseases; 1-9.
APPENDIX 1: TABLE OF CLASSES OF VARIOUS
ANTIMICROBIAL DRUGS
APPENDIX 2: AUTHORIZATION FORM FOR DATA
COLLECTION
| 

