5.1.7.1-ethyl-3-methylimidazolium
trifluoromethanesulfonate, [EMIM] [TfO]
Table 5-22: Activity coefficients at infinite
dilution of organic solutes in 1-ethyl-3-
methylimidazolium trifluoromethanesulfonate with n3 =
8.01 mmol (29.3 %)
at T = (313.15, 323.15 and 333.15) K.
Experimental at /K
Solute
|
n3/mmol
|
T=313.15
|
T=323.15
|
T=333.15
|
n-pentane
|
8.01
|
40.74
|
38.62
|
36.57
|
n-hexane
|
8.01
|
66.98
|
63.11
|
60.16
|
n-heptane
|
8.01
|
107.28
|
99.46
|
93.32
|
n-octane
|
8.01
|
170.38
|
154.86
|
143.78
|
Hex-1-ene
|
8.01
|
28.60
|
27.68
|
26.72
|
Hept-1-ene
|
8.01
|
47.01
|
44.82
|
42.87
|
Oct-1-ene
|
8.01
|
75.66
|
70.99
|
67.26
|
Non-1-ene
|
8.01
|
122.18
|
113.25
|
105.70
|
Dec-1-ene
|
8.01
|
171.88
|
162.96
|
154.72
|
Undec-1-ene
|
8.01
|
315.79
|
283.50
|
257.07
|
Pent-1-yne
|
8.01
|
3.95
|
4.02
|
4.08
|
Hex-1-yne
|
8.01
|
6.35
|
6.43
|
6.47
|
Hept-1-yne
|
8.01
|
9.83
|
9.79
|
9.78
|
Oct-1-yne
|
8.01
|
16.32
|
16.24
|
16.07
|
Non-1-yne
|
8.01
|
23.77
|
24.07
|
24.14
|
Cyclopentane
|
8.01
|
20.92
|
20.00
|
18.99
|
Cyclohexane
|
8.01
|
33.30
|
31.24
|
29.29
|
Cycloheptane
|
8.01
|
46.87
|
43.29
|
40.23
|
Cyclooctane
|
8.01
|
67.24
|
61.17
|
56.18
|
Methanol
|
8.01
|
0.73
|
0.70
|
0.67
|
Ethanol
|
8.01
|
1.19
|
1.12
|
1.06
|
Benzene
|
8.01
|
2.23
|
2.25
|
2.26
|
Toluene
|
8.01
|
3.62
|
3.65
|
3.67
|
Ethylbenzene
|
8.01
|
6.37
|
6.32
|
6.28
|
|
Table 5-23: Activity coefficients at infinite
dilution of organic solutes in 1-ethyl-3-
methylimidazolium trifluoromethanesulfonate with n3 =
6.23 mmol (32.88 %)
at T = (313.15, 323.15 and 333.15) K.
|
Experimental at
|
/K
|
|
Solute
|
n3/mmol
|
T=313.15
|
T=323.15
|
T=333.15
|
n-pentane
|
6.23
|
42.50
|
39.52
|
36.94
|
n-hexane
|
6.23
|
67.09
|
65.82
|
61.25
|
n-heptane
|
6.23
|
102.33
|
94.91
|
90.16
|
n-octane
|
6.23
|
162.50
|
159.27
|
145.47
|
Hex-1-ene
|
6.23
|
29.47
|
27.76
|
27.84
|
Hept-1-ene
|
6.23
|
45.57
|
43.07
|
43.57
|
Oct-1-ene
|
6.23
|
74.91
|
68.50
|
64.18
|
Non-1-ene
|
6.23
|
126.58
|
117.25
|
106.34
|
Dec-1-ene
|
6.23
|
179.20
|
166.94
|
160.49
|
Undec-1-ene
|
6.23
|
320.33
|
288.38
|
260.50
|
Pent-1-yne
|
6.23
|
4.01
|
4.00
|
3.98
|
Hex-1-yne
|
6.23
|
6.36
|
6.51
|
6.76
|
Hept-1-yne
|
6.23
|
9.59
|
9.75
|
9.87
|
Oct-1-yne
|
6.23
|
15.56
|
16.56
|
17.03
|
Non-1-yne
|
6.23
|
24.02
|
24.13
|
24.36
|
Cyclopentane
|
6.23
|
21.22
|
20.92
|
19.50
|
Cyclohexane
|
6.23
|
34.13
|
32.13
|
30.31
|
Cycloheptane
|
6.23
|
45.37
|
43.95
|
38.46
|
Cyclooctane
|
6.23
|
69.71
|
62.49
|
55.74
|
Methanol
|
6.23
|
0.72
|
0.69
|
0.68
|
Ethanol
|
6.23
|
1.15
|
1.09
|
1.07
|
Benzene
|
6.23
|
2.21
|
2.23
|
2.28
|
Toluene
|
6.23
|
3.51
|
3.53
|
3.64
|
Ethylbenzene
|
6.23
|
6.03
|
6.22
|
6.46
|
|
Table 5-24: Average activity coefficients at
infinite dilution of organic solutes in 1-ethyl-
3-methylimidazolium trifluoromethanesulfonate at T =
(313.15, 323.15 and 333.15) K.
Experimental
|
at /K
|
|
Solute
|
T=313.15
|
T=323.15
|
T=333.15
|
n-pentane
|
41.62
|
39.07
|
36.76
|
n-hexane
|
67.04
|
64.47
|
60.71
|
n-heptane
|
104.81
|
97.19
|
91.74
|
n-octane
|
166.44
|
157.07
|
144.63
|
Hex-1-ene
|
29.04
|
27.72
|
27.28
|
Hept-1-ene
|
46.29
|
43.95
|
43.22
|
Oct-1-ene
|
75.29
|
69.75
|
65.72
|
Non-1-ene
|
124.38
|
115.25
|
106.02
|
Dec-1-ene
|
175.54
|
164.95
|
157.61
|
Undec-1-ene
|
318.06
|
285.94
|
258.79
|
Pent-1-yne
|
3.98
|
4.01
|
4.03
|
Hex-1-yne
|
6.36
|
6.47
|
6.62
|
Hept-1-yne
|
9.71
|
9.77
|
9.83
|
Oct-1-yne
|
15.94
|
16.40
|
16.55
|
Non-1-yne
|
23.81
|
24.10
|
24.25
|
Cyclopentane
|
21.07
|
20.46
|
19.25
|
Cyclohexane
|
33.72
|
31.69
|
29.80
|
Cycloheptane
|
46.12
|
43.62
|
39.35
|
Cyclooctane
|
68.48
|
61.83
|
55.96
|
Methanol
|
0.73
|
0.70
|
0.68
|
Ethanol
|
1.17
|
1.11
|
1.07
|
Benzene
|
2.22
|
2.24
|
2.27
|
Toluene
|
3.57
|
3.59
|
3.66
|
Ethylbenzene
|
6.20
|
6.27
|
6.37
|
|
Table 5-25: Excess molar enthalpies at
infinite dilution of organic solutes for the ionic
liquid 1-ethyl-3-methylimidazolium trifluoromethanesulfonate,
calculated using the Gibbs-
Helmholtz equation.
SOLUTE
|
Linear regression using Eq.(2-11)
|
|
|
|
|
n-pentane
|
0.648
|
1.659
|
0.999
|
5.39
|
n-hexane
|
0.516
|
2.561
|
0.981
|
4.29
|
n-heptane
|
0.695
|
2.430
|
0.997
|
5.78
|
n-octane
|
0.731
|
2.784
|
0.986
|
6.08
|
Hex-1-ene
|
0.327
|
2.321
|
0.936
|
2.72
|
Hept-1-ene
|
0.360
|
2.681
|
0.928
|
2.99
|
Oct-1-ene
|
0.709
|
2.054
|
0.997
|
5.90
|
Non-1-ene
|
0.832
|
2.167
|
0.998
|
6.92
|
Dec-1-ene
|
0.563
|
3.368
|
0.994
|
4.68
|
Undec-1-ene
|
1.076
|
2.327
|
1.000
|
8.94
|
Pent-1-yne
|
-0.065
|
1.590
|
0.990
|
-0.54
|
Hex-1-yne
|
-0.209
|
2.515
|
0.994
|
-1.74
|
Hept-1-yne
|
-0.061
|
2.469
|
0.999
|
-0.51
|
Oct-1-yne
|
-0.197
|
3.401
|
0.928
|
-1.64
|
Non-1-yne
|
-0.096
|
3.477
|
0.973
|
-0.80
|
Cyclopentane
|
0.471
|
1.550
|
0.953
|
3.91
|
Cyclohexane
|
0.644
|
1.463
|
0.999
|
5.35
|
Cycloheptane
|
0.826
|
1.202
|
0.965
|
6.87
|
Cyclooctane
|
1.053
|
0.866
|
0.999
|
8.75
|
Methanol
|
0.373
|
-1.516
|
0.992
|
3.10
|
Ethanol
|
0.491
|
-1.415
|
0.989
|
4.09
|
Benzene
|
-0.116
|
1.668
|
0.983
|
-0.96
|
Toluene
|
-0.129
|
1.683
|
0.931
|
-1.08
|
Ethylbenzene
|
-0.141
|
2.273
|
0.987
|
-1.17
|
|
Chapter 5: Results
6
5
4
3
2.95 3 3.05 3.1 3.15 3.2 3.25
1000K/T
1n( L13)
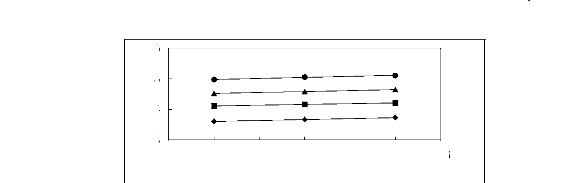
Figure 5-41: Plots of versus for alkanes in
[EMIM] [TfO] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
n-pentane, () n-hexane, (?)
n-heptane and (?) n-octane.
6
5
4
3
2.95 3 3.05 3.1 3.15 3.2 3.25
1000K/T
In( EP13)
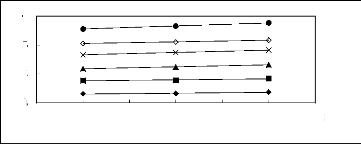
Figure 5-42: Plots of versus for alk-1-nes in
[EMIM] [TfO] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
hex-1-ene, () hept-1-ene,
(?) oct-1-ene, (x) non-1-ene, () dec-1-ene and (?)
undec-1-ene.
2.95 3 3.05 3.1 3.15 3.2 3.25
1000K/T
Figure 5-43: Plots of versus for alk-1-ynes in
[EMIM] [TfO] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
pent-1-yne, () hex-1-yne,
(?) hept-1-yne, (?) oct-1-yne and () non-1-yne.
2.95 3 3.05 3.1 3.15 3.2 3.25
1000K/T
In( EF13)
3
2
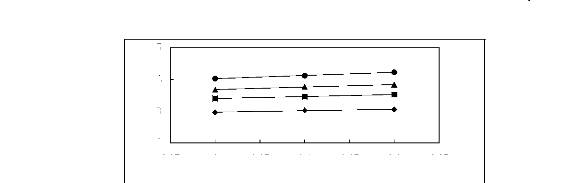
Figure 5-44: Plots of versus for cycloalkanes in
[EMIM] [TfO] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
cyclopentane, () cyclohexane,
(?) cycloheptane and (?) cyclooctane.
2.95 3 3.05 3.1 3.15 3.2 3.25
1000K/T
In( EF13)
-0.1
-0.2
-0.3
-0.4
-0.5
0.2
0.1
0
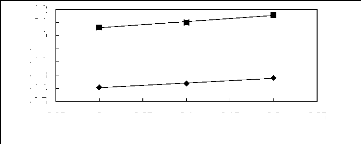
Figure 5-45: Plots of versus for alkanols in
[EMIM] [TfO] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
methanol and () ethanol.
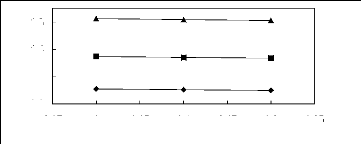
2.95 3 3.05 3.1 3.15 3.2 3.25
1000K/T
ln( L13)
0.6
1.8
1.4
1
Figure 5-46: Plots of versus for alkylbenzenes
in [EMIM] [TfO] together with a
linear correlation of the data using the Gibbs-Helmholtz
equation; () benzene, () toluene and
(?) ethylbenzene.
Chapter 5: Results
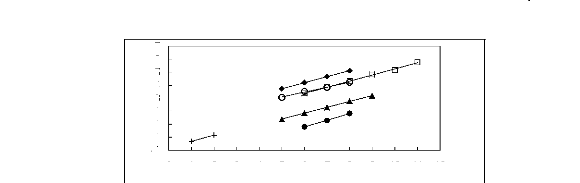
0 1 2 3 4 5 6 7 8 9 10 11 12
Nc
7
6
5
4
in(L13)
3
2
1
0
-1
Figure 5-47: Plots of versus the number of
carbon atoms at 313.15 K for () n-alkanes,
(?) alk-1-enes, (?) alk-1-ynes, and (?) cycloalkanes, (+)
alkanols and (?) alkylbenzenes in
[EMIM] [TfO].
| 


