5.1.6. 1-Butyl-3-methylimidazolium hexafluoroantimonate,
[BMIM] [SbF6]
Table 5-18: Activity coefficients at infinite
dilution of organic solutes in
1-butyl-3-methylimidazolium hexafluoroantimonate with
n3 = 3.312 mmol (26.90 %) at T =
(313.15, 323.15 and
333.15) K.
|
Experimental at
|
/K
|
|
Solute
|
n3/mmol
|
T=313.15
|
T=323.15
|
T=333.15
|
n-pentane.
|
3.312
|
19.611
|
17.552
|
16.962
|
n-hexane
|
3.312
|
28.627
|
26.428
|
25.994
|
n-heptane
|
3.312
|
37.066
|
34.182
|
31.424
|
n-octane
|
3.312
|
40.437
|
39.454
|
39.176
|
n-nonane
|
3.312
|
47.704
|
44.947
|
44.137
|
Pent-1-ene
|
3.312
|
9.956
|
10.168
|
9.938
|
Hex-1-ene
|
3.312
|
14.006
|
13.017
|
13.356
|
Hept-1-ene
|
3.312
|
19.291
|
17.762
|
15.857
|
Oct-1-ene
|
3.312
|
25.680
|
24.604
|
24.205
|
Non-1-ene
|
3.312
|
32.427
|
30.780
|
30.800
|
Pent-1-yne
|
3.312
|
2.817
|
2.885
|
2.933
|
Hex-1-yne
|
3.312
|
4.256
|
4.108
|
4.102
|
Hept-1-yne
|
3.312
|
5.741
|
5.816
|
5.739
|
Oct-1-yne
|
3.312
|
8.678
|
8.820
|
8.558
|
Non-1-yne
|
3.312
|
11.426
|
10.934
|
10.894
|
Cyclopentane
|
3.312
|
11.379
|
10.560
|
10.193
|
Cyclohexane
|
3.312
|
16.409
|
11.044
|
14.132
|
Cycloheptane
|
3.312
|
19.954
|
15.745
|
17.295
|
Cyclooctane
|
3.312
|
22.852
|
17.172
|
20.433
|
Methanol
|
3.312
|
1.701
|
1.639
|
1.565
|
Ethanol
|
3.312
|
2.334
|
2.144
|
2.005
|
Propan-1-ol
|
3.312
|
3.128
|
2.819
|
2.513
|
Benzene
|
3.312
|
1.229
|
1.276
|
1.314
|
Toluene
|
3.312
|
1.866
|
1.876
|
1.916
|
Ethylbenzene
|
3.312
|
3.113
|
3.085
|
3.176
|
Acetone
|
3.312
|
0.403
|
0.445
|
0.463
|
Butan-2-one
|
3.312
|
0.648
|
0.681
|
0.702
|
|
Table 5-19: Activity coefficients at infinite
dilution of organic solutes in
1-butyl-3-methylimidazolium hexafluoroantimonate with
n3 = 4.578 mmol (31.98 %) at T =
(313.15, 323.15 and
333.15) K.
Experimental at /K
Solute
|
n3/mmol
|
T=313.15
|
T=323.15
|
T=333.15
|
n-pentane.
|
4.578
|
21.459
|
18.268
|
18.088
|
n-hexane
|
4.578
|
27.893
|
28.232
|
26.626
|
n-heptane
|
4.578
|
38.734
|
35.798
|
34.386
|
n-octane
|
4.578
|
44.603
|
42.656
|
42.714
|
n-nonane
|
4.578
|
48.766
|
49.123
|
47.263
|
Pent-1-ene
|
4.578
|
10.474
|
10.232
|
10.442
|
Hex-1-ene
|
4.578
|
13.134
|
14.103
|
13.754
|
Hept-1-ene
|
4.578
|
21.109
|
19.698
|
16.623
|
Oct-1-ene
|
4.578
|
25.700
|
25.696
|
24.915
|
Non-1-ene
|
4.578
|
32.573
|
33.420
|
32.620
|
Pent-1-yne
|
4.578
|
2.883
|
2.885
|
3.027
|
Hex-1-yne
|
4.578
|
4.054
|
4.232
|
4.318
|
Hept-1-yne
|
4.578
|
6.109
|
6.084
|
6.231
|
Oct-1-yne
|
4.578
|
9.402
|
9.080
|
8.992
|
Non-1-yne
|
4.578
|
11.634
|
11.846
|
11.646
|
Cyclopentane
|
4.578
|
11.881
|
10.860
|
10.657
|
Cyclohexane
|
4.578
|
15.391
|
18.626
|
14.558
|
Cycloheptane
|
4.578
|
18.666
|
21.075
|
18.175
|
Cyclooctane
|
4.578
|
23.418
|
27.128
|
21.467
|
Methanol
|
4.578
|
1.880
|
1.711
|
1.565
|
Ethanol
|
4.578
|
2.456
|
2.296
|
2.065
|
Propan-1-ol
|
4.578
|
3.222
|
2.961
|
2.747
|
Benzene
|
4.578
|
1.311
|
1.374
|
1.370
|
Toluene
|
4.578
|
1.784
|
1.944
|
1.972
|
Ethylbenzene
|
4.578
|
2.917
|
3.225
|
3.450
|
Acetone
|
4.578
|
0.437
|
0.459
|
0.487
|
Butan-2-one
|
4.578
|
0.632
|
0.681
|
0.718
|
|
Table 5-20: Average activity coefficients at
infinite dilution of organic solutes in
1-butyl-3-methylimidazolium hexafluoroantimonate at T
= (313.15, 323.15 and 333.15) K.
|
Experimental
|
at /K
|
|
Solute
|
T=313.15
|
T=323.15
|
T=333.15
|
n-pentane.
|
20.535
|
17.910
|
17.525
|
n-hexane
|
28.260
|
27.330
|
26.310
|
n-heptane
|
37.900
|
34.990
|
32.905
|
n-octane
|
42.520
|
41.055
|
40.945
|
n-nonane
|
48.235
|
47.035
|
45.700
|
Pent-1-ene
|
10.215
|
10.200
|
10.190
|
Hex-1-ene
|
13.570
|
13.560
|
13.555
|
Hept-1-ene
|
20.200
|
18.730
|
16.240
|
Oct-1-ene
|
25.690
|
25.150
|
24.560
|
Non-1-ene
|
32.500
|
32.100
|
31.710
|
Pent-1-yne
|
2.850
|
2.885
|
2.980
|
Hex-1-yne
|
4.155
|
4.170
|
4.210
|
Hept-1-yne
|
5.925
|
5.950
|
5.985
|
Oct-1-yne
|
9.040
|
8.950
|
8.775
|
Non-1-yne
|
11.530
|
11.390
|
11.270
|
Cyclopentane
|
11.630
|
10.710
|
10.425
|
Cyclohexane
|
15.900
|
14.835
|
14.345
|
Cycloheptane
|
19.310
|
18.410
|
17.735
|
Cyclooctane
|
23.135
|
22.150
|
20.950
|
Methanol
|
1.790
|
1.675
|
1.565
|
Ethanol
|
2.395
|
2.220
|
2.035
|
Propan-1-ol
|
3.175
|
2.890
|
2.630
|
Benzene
|
1.270
|
1.325
|
1.342
|
Toluene
|
1.825
|
1.910
|
1.944
|
Ethylbenzene
|
3.015
|
3.155
|
3.313
|
Acetone
|
0.420
|
0.452
|
0.475
|
Butan-2-one
|
0.640
|
0.681
|
0.710
|
|
Table 5-21: Excess molar enthalpies at
infinite dilution of organic solutes in the ionic
liquid 1-butyl-3-methylimidazolium hexafluoroantimonate
calculated using the Gibbs-
Helmholtz equation.
Solute
|
Linear regression using Eq.(2-21)
|
|
|
|
|
n-pentane.
|
0.793
|
0.467
|
0.851
|
6.59
|
n-hexane
|
0.357
|
2.198
|
0.999
|
2.97
|
n-heptane
|
0.707
|
1.371
|
0.994
|
5.88
|
n-octane
|
0.189
|
3.141
|
0.803
|
1.57
|
n-nonane
|
0.270
|
3.013
|
0.999
|
2.25
|
Pent-1-ene
|
0.012
|
2.285
|
0.987
|
0.10
|
Hex-1-ene
|
0.006
|
2.590
|
0.964
|
0.05
|
Hept-1-ene
|
1.091
|
-0.474
|
0.969
|
9.07
|
Oct-1-ene
|
0.225
|
2.527
|
0.999
|
1.87
|
Non-1-ene
|
0.123
|
3.088
|
1.000
|
1.02
|
Pent-1-yne
|
-0.223
|
1.758
|
0.936
|
-1.85
|
Hex-1-yne
|
-0.066
|
1.634
|
0.936
|
-0.55
|
Hept-1-yne
|
-0.050
|
1.940
|
0.991
|
-0.42
|
Oct-1-yne
|
0.149
|
1.727
|
0.966
|
1.24
|
Non-1-yne
|
0.114
|
2.080
|
0.998
|
0.95
|
Cyclopentane
|
0.547
|
0.694
|
0.921
|
4.55
|
Cyclohexane
|
0.515
|
1.114
|
0.961
|
4.28
|
Cycloheptane
|
0.425
|
1.598
|
0.995
|
3.53
|
Cyclooctane
|
0.496
|
1.556
|
0.995
|
4.12
|
Methanol
|
0.672
|
-1.567
|
1.000
|
5.59
|
Ethanol
|
0.814
|
-1.731
|
0.998
|
6.77
|
Propan-1-ol
|
0.942
|
-1.858
|
1.000
|
7.83
|
Benzene
|
-0.276
|
1.126
|
0.912
|
-2.30
|
Toluene
|
-0.316
|
1.617
|
0.939
|
-2.63
|
Ethylbenzene
|
-0.471
|
2.611
|
0.999
|
-3.92
|
Acetone
|
-0.615
|
1.105
|
0.998
|
-5.11
|
Butan-2-one
|
-0.519
|
1.218
|
0.987
|
-4.32
|
|
3.9
3.7
In( 0'13)
3.5
3.3
3.1
2.9
2.7
2.95 3 3.05 3.1 3.15 3.2 3.25
1000K/T
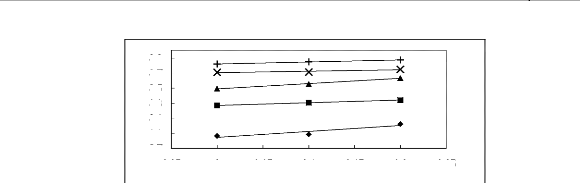
Figure 5-33: Plots of versus for alkanes in
[BMIM] [SbF6] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
n-pentane, () n-hexane,
(?) n-heptane, (x) n-octane, (+) n-nonane.
2.95 3 3.05 3.1 3.15 3.2 3.25
1000K/T
1n( L13)
2.8
2.6
2.4
2.2
3.6
3.4
3.2
2
3
Figure 5-34: Plots of versus for alk-1-enes in
[BMIM] [SbF6] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
pent-1-ene, () hex-1-ene,
(?) hept-1-ene, (x) oct-1-ene and (+) non-1-ene
2.95 3 3.05 3.1 3.15 3.2 3.25
1000K/T
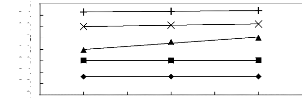
Figure 5-35: Plots of versus for alk-1-ynes in
[BMIM] [SbF6] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
pent-1-yne, () hex-1-yne,
(?) hept-1-yne, (x) oct-1-yne and (?)
non-1-yne.
2.95 3 3.05 3.1 3.15 3.2 3.25
1000K/T
3.4
3.2
3
111( EF13)
2.8
2.6
2.4
2.2
2
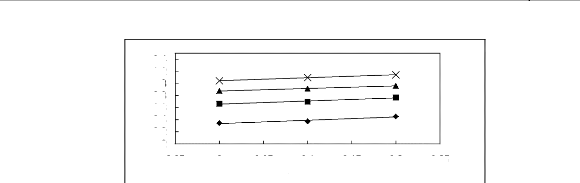
Figure 5-36: Plots of versus for cycloalkanes in
[BMIM] [SbF6] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
cyclopentane, () cyclohexane,
(?) cycloheptane and (x) cyclooctane.
111( EF13)
0.95
0.75
0.55
0.35
1.15
2.95 3 3.05 3.1 3.15 3.2 3.25
1000K/T
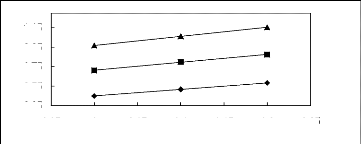
Figure 5-37: Plots of versus for alkanols in
[BMIM] [SbF6] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
methanol, () ethanol and
(?) propan-1-ol.
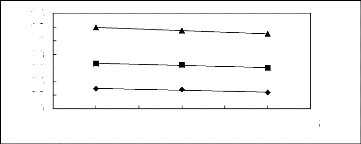
1.4
1.2
1
0.8
0.6
0.4
0.2
0
2.95 3 3.05 3.1 3.15 3.2 3.25
1000K/T
In( 0'13)
Figure 5-38: Plots of versus for alkylbenzenes
in [BMIM] [SbF6] together with a
linear correlation of the data using the Gibbs-Helmholtz
equation; () benzene, () toluene and
(?) ethylbenzene.
2.95 3 3.05 3.1 3.15 3.2 3.25
1000K/T
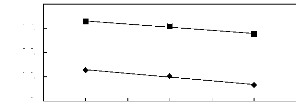
-0.2
-0.4
in( L1:13)
-0.6
-0.8
-1
Figure 5-39: Plots of versus for ketones in
[BMIM] [SbF6] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
acetone and () butan-2-one.
0 1 2 3 4 5 6 7 8 9 10
Nc
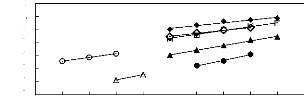
in (L13)
-1
-2
4
2
3
0
5
1
Figure 5-40: Plots of versus the number of
carbon atoms at 313.15 K for () n-alkanes,
(?) alk-1-enes, (?) alk-1-ynes, and () cycloalkanes, (o)
alkanols, (?) alkylbenzenes and (?)
ketones in [BMIM] [SbF6].
| 


