5.1.3. Trihexyltetradecylphosphonium tetrafluoroborate,
[3C6C14P] [BF4]
Table 5-6: Activity coefficients at infinite
dilution of organic solutes in
trihexyltetradecylphosphonium tetrafluoroborate with solvent
column loading
n3 = 2.395 mmol (25.09 %) at T = (313.15,
333.15,
|
353.15 and 373.15) K.
|
|
|
Experimental
|
at /K
|
|
|
Solute
|
n3/ mmol
|
T=313.15
|
T=333.15
|
T=353.15
|
T=373.15
|
n-pentane.
|
2.395
|
1.231
|
1.211
|
1.208
|
1.199
|
n-hexane
|
2.395
|
1.392
|
1.368
|
1.362
|
1.353
|
n-heptane
|
2.395
|
1.579
|
1.538
|
1.525
|
1.516
|
n-octane
|
2.395
|
1.792
|
1.769
|
1.711
|
1.697
|
n-nonane
|
2.395
|
2.053
|
1.965
|
1.936
|
1.912
|
n-decane
|
2.395
|
2.400
|
2.236
|
2.184
|
2.125
|
Pent-1-ene
|
2.395
|
0.971
|
0.984
|
0.992
|
1.017
|
Hex-1-ene
|
2.395
|
1.117
|
1.120
|
1.125
|
1.131
|
Hept-1-ene
|
2.395
|
1.247
|
1.250
|
1.254
|
1.262
|
Oct-1-ene
|
2.395
|
1.403
|
1.412
|
1.413
|
1.419
|
Non-1-ene
|
2.395
|
1.630
|
1.589
|
1.568
|
1.596
|
Pent-1-yne
|
2.395
|
0.569
|
0.591
|
0.624
|
0.690
|
Hex-1-yne
|
2.395
|
0.610
|
0.646
|
0.682
|
0.705
|
Hept-1-yne
|
2.395
|
0.670
|
0.697
|
0.749
|
0.820
|
Octy-1-ne
|
2.395
|
0.760
|
0.818
|
0.870
|
0.923
|
Non-1-yne
|
2.395
|
0.784
|
0.871
|
0.950
|
1.025
|
Cyclopentane
|
2.395
|
0.841
|
0.816
|
0.813
|
0.802
|
Cyclohexane
|
2.395
|
0.970
|
0.935
|
0.919
|
0.905
|
Cycloheptane
|
2.395
|
1.047
|
1.007
|
0.982
|
0.965
|
Cyclooctane
|
2.395
|
1.112
|
1.088
|
1.073
|
1.063
|
Methanol
|
2.395
|
0.548
|
0.500
|
0.466
|
0.453
|
Ethanol
|
2.395
|
0.611
|
0.553
|
0.507
|
0.479
|
Benzene
|
2.395
|
0.432
|
0.440
|
0.447
|
0.464
|
Toluene
|
2.395
|
0.524
|
0.539
|
0.546
|
0.564
|
Ethylbenzene
|
2.395
|
0.659
|
0.665
|
0.682
|
0.696
|
Acetone
|
2.395
|
0.436
|
0.437
|
0.438
|
0.439
|
Butan-2-one
|
2.395
|
0.450
|
0.452
|
0.455
|
0.457
|
|
Table 5-7: Activity coefficients at infinite
dilution of organic solutes in
trihexyltetradecylphosphonium tetrafluoroborate with solvent
column loading
n3 = 2.236 mmol (30.97 %) at T = (313.15,
333.15,
|
353.15 and 373.15) K.
|
|
|
Experimental
|
at /K
|
|
|
Solute
|
n3/ mmol
|
T=313.15
|
T=333.15
|
T=353.15
|
T=373.15
|
n-pentane
|
2.694
|
1.285
|
1.269
|
1.230
|
1.193
|
n-hexane
|
2.694
|
1.408
|
1.400
|
1.358
|
1.311
|
n-heptane
|
2.694
|
1.577
|
1.590
|
1.527
|
1.458
|
n-octane
|
2.694
|
1.788
|
1.729
|
1.701
|
1.623
|
n-nonane
|
2.694
|
2.069
|
1.991
|
1.912
|
1.812
|
n-decane
|
2.694
|
2.360
|
2.290
|
2.172
|
2.069
|
Pent-1-ene
|
2.694
|
1.017
|
0.990
|
0.958
|
0.929
|
Hex-1-ene
|
2.694
|
1.101
|
1.090
|
1.083
|
1.073
|
Hept-1-ene
|
2.694
|
1.273
|
1.248
|
1.234
|
1.192
|
Oct-1-ene
|
2.694
|
1.449
|
1.402
|
1.393
|
1.345
|
Non-1-ene
|
2.694
|
1.640
|
1.607
|
1.580
|
1.476
|
Pent-1-yne
|
2.694
|
0.599
|
0.611
|
0.638
|
0.594
|
Hex-1-yne
|
2.694
|
0.596
|
0.650
|
0.668
|
0.697
|
Hept-1-yne
|
2.694
|
0.806
|
0.789
|
0.793
|
0.746
|
Oct-1-yne
|
2.694
|
0.738
|
0.820
|
0.838
|
0.839
|
Non-1-yne
|
2.694
|
0.862
|
0.871
|
0.894
|
0.931
|
Cyclopentane
|
2.694
|
0.845
|
0.838
|
0.809
|
0.812
|
Cyclohexane
|
2.694
|
0.980
|
0.947
|
0.887
|
0.883
|
Cycloheptane
|
2.694
|
0.993
|
0.965
|
0.952
|
0.931
|
Cyclooctane
|
2.694
|
1.248
|
1.160
|
1.077
|
1.011
|
Methanol
|
2.694
|
0.526
|
0.482
|
0.446
|
0.381
|
Ethanol
|
2.694
|
0.547
|
0.513
|
0.495
|
0.423
|
Benzene
|
2.694
|
0.388
|
0.410
|
0.437
|
0.432
|
Toluene
|
2.694
|
0.528
|
0.523
|
0.574
|
0.584
|
Ethylbenzene
|
2.694
|
0.661
|
0.677
|
0.686
|
0.734
|
Acetone
|
2.694
|
0.426
|
0.427
|
0.428
|
0.431
|
Butan-2-one
|
2.694
|
0.438
|
0.442
|
0.447
|
0.451
|
|
Table 5-8: Average activity coefficients at
infinite dilution of organic solutes in
trihexyltetradecylphosphonium tetrafluoroborate at T =
(313.15, 333.15, 353.15 and 373.15) K.
|
|
Experimental at
|
/K
|
|
Solute
|
T=313.15
|
T=333.15
|
T=353.15
|
T=373.15
|
n-pentane.
|
1.258
|
1.240
|
1.219
|
1.196
|
n-hexane
|
1.400
|
1.384
|
1.360
|
1.332
|
n-heptane
|
1.578
|
1.564
|
1.526
|
1.487
|
n-octane
|
1.790
|
1.749
|
1.706
|
1.660
|
n-nonane
|
2.061
|
1.978
|
1.924
|
1.862
|
n-decane
|
2.380
|
2.263
|
2.178
|
2.097
|
Pent-1-ene
|
0.994
|
0.987
|
0.975
|
0.973
|
Hex-1-ene
|
1.109
|
1.105
|
1.104
|
1.102
|
Hept-1-ene
|
1.260
|
1.249
|
1.244
|
1.227
|
Oct-1-ene
|
1.426
|
1.407
|
1.403
|
1.382
|
Non-1-ene
|
1.635
|
1.598
|
1.574
|
1.536
|
Pent-1-yne
|
0.584
|
0.601
|
0.631
|
0.642
|
Hex-1-yne
|
0.603
|
0.648
|
0.675
|
0.701
|
Hept-1-yne
|
0.738
|
0.743
|
0.771
|
0.783
|
Oct-1-yne
|
0.749
|
0.819
|
0.854
|
0.881
|
Non-1-yne
|
0.823
|
0.871
|
0.922
|
0.978
|
Cyclopentane
|
0.843
|
0.827
|
0.811
|
0.807
|
Cyclohexane
|
0.975
|
0.941
|
0.903
|
0.894
|
Cycloheptane
|
1.020
|
0.986
|
0.967
|
0.948
|
Cyclooctane
|
1.180
|
1.124
|
1.075
|
1.037
|
Methanol
|
0.537
|
0.491
|
0.456
|
0.417
|
Ethanol
|
0.579
|
0.533
|
0.501
|
0.451
|
Benzene
|
0.410
|
0.425
|
0.442
|
0.448
|
Toluene
|
0.526
|
0.531
|
0.560
|
0.574
|
Ethylbenzene
|
0.660
|
0.671
|
0.684
|
0.715
|
Acetone
|
0.431
|
0.432
|
0.433
|
0.435
|
Butan-2-one
|
0.444
|
0.447
|
0.451
|
0.454
|
|
Table 5-9: Partial molar excess enthalpies at
infinite dilution for organic solutes in the
ionic liquid trihexyltetradecylphosphonium tetrafluoroborate,
calculated from the Gibbs-
Helmholtz equation.
SOLUTE
|
Linear regression using Eq.(2-11)
|
|
|
|
n-pentane.
|
0.096
|
-0.077
|
0.984
|
0.80
|
n-hexane
|
0.095
|
0.035
|
0.964
|
0.79
|
n-heptane
|
0.115
|
0.093
|
0.933
|
0.96
|
n-octane
|
0.144
|
0.123
|
0.989
|
1.20
|
n-nonane
|
0.192
|
0.108
|
0.997
|
1.60
|
n-decane
|
0.241
|
0.093
|
0.999
|
2.00
|
Pent-1-ene
|
0.044
|
-0.149
|
0.952
|
0.37
|
Hex-1-ene
|
0.018
|
0.065
|
0.949
|
0.10
|
Hept-1-ene
|
0.048
|
0.077
|
0.939
|
0.40
|
Oct-1-ene
|
0.056
|
0.174
|
0.940
|
0.47
|
Non-1-ene
|
0.118
|
0.115
|
0.988
|
0.98
|
Pent-1-yne
|
-0.195
|
0.082
|
0.975
|
-1.62
|
Hex-1-yne
|
-0.290
|
0.425
|
0.986
|
-2.40
|
Hept-1-yne
|
-0.124
|
0.087
|
0.925
|
-1.03
|
Oct-1-yne
|
-0.312
|
0.720
|
0.960
|
-2.60
|
Non-1-yne
|
-0.335
|
0.870
|
0.996
|
-2.78
|
Cyclopentane
|
0.088
|
-0.455
|
0.966
|
0.74
|
Cyclohexane
|
0.177
|
-0.594
|
0.967
|
1.48
|
Cycloheptane
|
0.140
|
-0.431
|
0.990
|
1.17
|
Cyclooctane
|
0.253
|
-0.642
|
0.999
|
2.10
|
Methanol
|
0.486
|
-2.169
|
0.996
|
4.04
|
Ethanol
|
0.472
|
-2.046
|
0.981
|
3.92
|
Benzene
|
-0.179
|
-0.316
|
0.978
|
-1.49
|
Toluene
|
-0.183
|
-0.068
|
0.925
|
-1.52
|
Ethylbenzene
|
-0.149
|
0.054
|
0.913
|
-1.24
|
Acetone
|
-0.017
|
-0.786
|
0.946
|
-0.14
|
Butan-2-one
|
-0.044
|
-0.671
|
0.993
|
-0.37
|
|
Chapter 5: Results
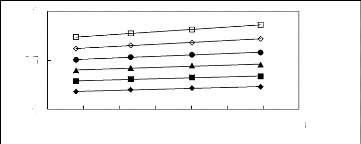
2.6 2.7 2.8 2.9 3 3.1 3.2 3.3
1000K/T
ln( LP 13)
0.5
0
1
Figure 5-9: Plots of versus for n-alkanes in
[3C6C14P] [BF4] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
n-pentane, () n-hexane,
(?) n-heptane, (?) n-octane, () n-nonane and (?)
n-decane.
|
0.6 0.5 0.4 0.3 0.2 0.1
0
-0.1
|
|
ln( LP 13)
|
|
|
|
|
2.6 2.8 3 3.2 3.4
1000K/T
Figure 5-10: Plots of versus for alk-1-enes in
[3C6C14P] [BF4] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
pent-1-ene, () hex-1-ene,
(?) hept-1ene, (?) oct-1-ene and () non-1-ene.
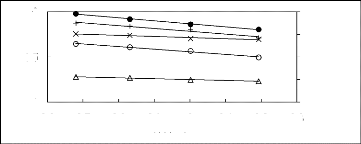
1n1 L13)
-0.5
-1
0
2.6 2.7 2.8 2.9 3 3.1 3.2 3.3
1000K/T
Figure 5-11: Plots of versus for alk-1-ynes in
[3C6C14P] [BF4] together with a linear
correlation of the data using the Gibbs-Helmholtz equation;
(?) pent-1-yne, (?) hex-1-yne,
(x) hept-1-yne, (+) oct-1-yne and (?)
non-1-yne.
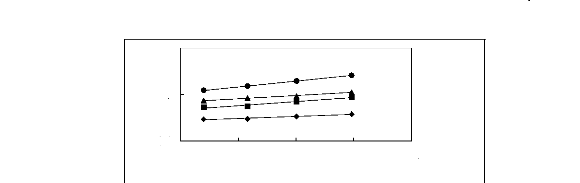
0.4
ln(1113)
0
-0.4
2.6 2.8 3 3.2 3.4
1000K/T
Figure 5-12: Plots of versus for cycloalkanes in
[3C6C14P] [BF4] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
cyclopentane, () cyclohexane,
(?) cycloheptane and (?) cyclooctane.
|
-0.4 -0.5 -0.6 -0.7 -0.8 -0.9
|
|
ln(1313)
|
|
|
|
|
2.6 2.8 3 3.2 3.4
1000K/T
Figure 5-13: Plots of versus for alkanols in
[3C6C14P] [BF4] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; (?)
methanol and (?) ethanol.
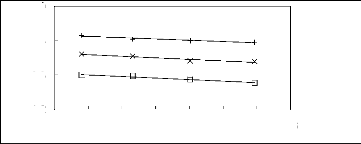
ln(111:13)
-0.4
-0.8
-1.2
0
2.6 2.7 2.8 2.9 3 3.1 3.2 3.3
1000K/T
Figure 5-14: Plots of versus for alkylbenzenes
in [3C6C14P] [BF4] together with a
linear correlation of the data using the Gibbs-Helmholtz
equation; (?) benzene, (x) toluene and
(+) ethylbenzene.
Figure 5-15: Plots of versus for ketones in
[3C6C14P] [BF4] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; (?)
acetone and (?) butan-2-one.
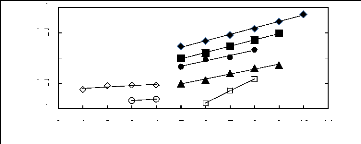
ln(119:13)
-0.5
0.5
-1
0
1
0 1 2 3 4 5 6 7 8 9 10 11
Number of carbon atoms, Nc
Figure 5-16: Plots of versus the number of
carbon atoms at 313.15 K for () n-alkanes,
() alk-1-enes, (?) alk-1-ynes, (?) cycloalkanes, () alkanols,
(?) alkylbenzenes and
(?) ketones in [3C6C14P] [BF4].
5.1.4. Trihexyltetradecylphosphonium
hexafluorophosphate, [3C6C14P] [PF6] Table 5-10: Activity
coefficients at infinite dilution of organic solutes in
trihexyltetradecylphosphonium hexafluorophosphate with
n3 = 1.615 mmol (25.1 %) at T =
(313.15, 333.15, 353.15
and 363.15) K.
|
Experimental
|
at /K
|
|
|
Solute
|
n3/mmol
|
T=313.15
|
T=333.15
|
T=353.15
|
T=363.15
|
n-pentane.
|
1.615
|
1.773
|
1.517
|
1.268
|
1.193
|
n-hexane
|
1.615
|
1.960
|
1.687
|
1.482
|
1.430
|
n-heptane
|
1.615
|
2.246
|
1.806
|
1.648
|
1.555
|
n-octane
|
1.615
|
2.528
|
2.120
|
1.869
|
1.812
|
n-nonane
|
1.615
|
2.774
|
2.426
|
2.088
|
1.984
|
n-decane
|
1.615
|
3.278
|
2.620
|
2.295
|
2.186
|
Pent-1-ene
|
1.615
|
1.421
|
1.236
|
1.066
|
1.049
|
Hex-1-ene
|
1.615
|
1.665
|
1.410
|
1.138
|
1.128
|
Hept-1-ene
|
1.615
|
1.857
|
1.581
|
1.321
|
1.269
|
Oct-1-ene
|
1.615
|
2.023
|
1.752
|
1.514
|
1.437
|
Non-1-ene
|
1.615
|
2.400
|
2.013
|
1.617
|
1.539
|
Dec-1-ene
|
1.615
|
2.788
|
2.276
|
1.827
|
1.750
|
Pent-1-yne
|
1.615
|
1.025
|
0.549
|
0.697
|
0.668
|
Hex-1-yne
|
1.615
|
1.145
|
0.649
|
0.780
|
0.732
|
Hept-1-yne
|
1.615
|
1.216
|
0.630
|
0.833
|
0.798
|
Oct-1-yne
|
1.615
|
1.369
|
0.754
|
0.962
|
0.928
|
Non-1-yne
|
1.615
|
1.476
|
0.827
|
1.063
|
0.978
|
Dec-1-yne
|
1.615
|
1.595
|
0.865
|
1.117
|
1.031
|
Cyclopentane
|
1.615
|
1.348
|
1.079
|
0.901
|
0.883
|
Cyclohexane
|
1.615
|
1.475
|
1.212
|
0.969
|
0.951
|
Cycloheptane
|
1.615
|
1.574
|
1.299
|
1.084
|
1.035
|
Cyclooctane
|
1.615
|
1.643
|
1.402
|
1.171
|
1.115
|
Cyclononane
|
1.615
|
1.883
|
1.567
|
1.319
|
1.234
|
Methanol
|
1.615
|
2.115
|
1.412
|
0.938
|
0.855
|
Ethanol
|
1.615
|
2.215
|
1.515
|
0.989
|
0.893
|
Benzene
|
1.615
|
0.659
|
0.543
|
0.438
|
0.415
|
Toluene
|
1.615
|
0.772
|
0.671
|
0.535
|
0.506
|
Ethylbenzene
|
1.615
|
0.961
|
0.783
|
0.630
|
0.626
|
Propylbenzene
|
1.615
|
1.103
|
0.949
|
0.779
|
0.735
|
Acetone
|
1.615
|
0.648
|
0.517
|
0.388
|
0.371
|
Butan-2-one
|
1.615
|
0.677
|
0.524
|
0.396
|
0.375
|
|
Table 5-11: Activity coefficients at infinite
dilution of organic solutes in
trihexyltetradecylphosphonium hexafluorophosphate with
n3 = 2.659 mmol (29.4 %) at T =
(313.15, 333.15, 353.15
and 363.15) K.
|
Experimental
|
at /K
|
|
|
Solute
|
n3/mmol
|
T=313.15
|
T=333.15
|
T=353.15
|
T=363.15
|
n-pentane.
|
2.659
|
1.921
|
1.553
|
1.288
|
1.251
|
n-hexane
|
2.659
|
2.072
|
1.795
|
1.496
|
1.472
|
n-heptane
|
2.659
|
2.194
|
2.004
|
1.668
|
1.651
|
n-octane
|
2.659
|
2.492
|
2.184
|
1.831
|
1.742
|
n-nonane
|
2.659
|
3.040
|
2.468
|
2.054
|
2.004
|
n-decane
|
2.659
|
3.324
|
2.896
|
2.309
|
2.236
|
Pent-1-ene
|
2.659
|
1.541
|
1.368
|
1.134
|
1.069
|
Hex-1-ene
|
2.659
|
1.701
|
1.436
|
1.264
|
1.174
|
Hept-1-ene
|
2.659
|
1.903
|
1.591
|
1.361
|
1.299
|
Oct-1-ene
|
2.659
|
2.199
|
1.794
|
1.484
|
1.433
|
Non-1-ene
|
2.659
|
2.496
|
1.975
|
1.627
|
1.549
|
Dec-1-ene
|
2.659
|
2.810
|
2.290
|
1.851
|
1.740
|
Pent-1-yne
|
2.659
|
1.061
|
0.861
|
0.713
|
0.654
|
Hex-1-yne
|
2.659
|
1.125
|
0.889
|
0.758
|
0.714
|
Hept-1-yne
|
2.659
|
1.250
|
1.034
|
0.831
|
0.822
|
Oct-1-yne
|
2.659
|
1.333
|
1.160
|
0.952
|
0.942
|
Non-1-yne
|
2.659
|
1.498
|
1.247
|
1.011
|
0.966
|
Dec-1-yne
|
2.659
|
1.615
|
1.315
|
1.063
|
1.009
|
Cyclopentane
|
2.659
|
1.344
|
1.141
|
0.941
|
0.871
|
Cyclohexane
|
2.659
|
1.459
|
1.234
|
1.049
|
0.991
|
Cycloheptane
|
2.659
|
1.602
|
1.335
|
1.100
|
1.049
|
Cyclooctane
|
2.659
|
1.799
|
1.442
|
1.199
|
1.143
|
Cyclononane
|
2.659
|
1.891
|
1.601
|
1.333
|
1.304
|
Methanol
|
2.659
|
2.139
|
1.444
|
0.984
|
0.881
|
Ethanol
|
2.659
|
2.315
|
1.491
|
1.015
|
0.911
|
Benzene
|
2.659
|
0.705
|
0.553
|
0.448
|
0.419
|
Toluene
|
2.659
|
0.814
|
0.639
|
0.541
|
0.516
|
Ethylbenzene
|
2.659
|
1.005
|
0.835
|
0.694
|
0.642
|
Propylbenzene
|
2.659
|
1.139
|
0.939
|
0.799
|
0.777
|
Acetone
|
2.659
|
0.694
|
0.507
|
0.404
|
0.381
|
Butan-2-one
|
2.659
|
0.735
|
0.542
|
0.410
|
0.373
|
|
Table 5-12: Average activity coefficients at
infinite dilution of organic solutes in
trihexyltetradecylphosphonium hexafluorophosphate
at T
= (313.15, 333.15, 353.15 and 363.15) K.
|
Experimental at
|
/K
|
|
Solute
|
T=313.15
|
T=333.15
|
T=353.15
|
T=363.15
|
n-pentane.
|
1.847
|
1.535
|
1.278
|
1.222
|
n-hexane
|
2.016
|
1.741
|
1.489
|
1.451
|
n-heptane
|
2.220
|
1.905
|
1.658
|
1.603
|
n-octane
|
2.510
|
2.152
|
1.850
|
1.777
|
n-nonane
|
2.907
|
2.447
|
2.071
|
1.994
|
n-decane
|
3.301
|
2.758
|
2.302
|
2.211
|
Pent-1-ene
|
1.481
|
1.302
|
1.100
|
1.059
|
Hex-1-ene
|
1.683
|
1.423
|
1.201
|
1.151
|
Hept-1-ene
|
1.880
|
1.586
|
1.341
|
1.284
|
Oct-1-ene
|
2.111
|
1.773
|
1.499
|
1.435
|
Non-1-ene
|
2.448
|
1.994
|
1.622
|
1.544
|
Dec-1-ene
|
2.799
|
2.283
|
1.839
|
1.745
|
Pent-1-yne
|
1.043
|
0.852
|
0.705
|
0.661
|
Hex-1-yne
|
1.135
|
0.898
|
0.769
|
0.723
|
Hept-1-yne
|
1.233
|
1.003
|
0.832
|
0.810
|
Oct-1-yne
|
1.351
|
1.161
|
0.957
|
0.935
|
Non-1-yne
|
1.487
|
1.250
|
1.037
|
0.972
|
Dec-1-yne
|
1.605
|
1.354
|
1.090
|
1.020
|
Cyclopentane
|
1.346
|
1.110
|
0.921
|
0.877
|
Cyclohexane
|
1.467
|
1.223
|
1.009
|
0.971
|
Cycloheptane
|
1.588
|
1.317
|
1.092
|
1.042
|
Cyclooctane
|
1.721
|
1.422
|
1.185
|
1.129
|
Cyclononane
|
1.887
|
1.584
|
1.326
|
1.269
|
Methanol
|
2.127
|
1.428
|
0.961
|
0.868
|
Ethanol
|
2.265
|
1.503
|
1.002
|
0.902
|
Benzene
|
0.682
|
0.548
|
0.443
|
0.417
|
Toluene
|
0.793
|
0.655
|
0.538
|
0.511
|
Ethylbenzene
|
0.983
|
0.809
|
0.662
|
0.634
|
Propylbenzene
|
1.121
|
0.944
|
0.789
|
0.756
|
Acetone
|
0.671
|
0.512
|
0.396
|
0.376
|
Butan-2-one
|
0.706
|
0.533
|
0.403
|
0.374
|
|
Table 5-13: Partial molar excess enthalpies at
infinite dilution for organic solutes in the
ionic liquid trihexyltetradecylphosphonium hexafluorophosphate
calculated from the Gibbs-
equation.
SOLUTE
|
Linear regression using Eq.(2-11)
|
|
|
|
|
n-pentane.
|
0.918
|
-2.325
|
0.999
|
7.63
|
n-hexane
|
0.742
|
-1.673
|
0.999
|
6.17
|
n-heptane
|
0.722
|
-1.516
|
0.999
|
6.00
|
n-octane
|
0.765
|
-1.528
|
0.999
|
6.36
|
n-nonane
|
0.839
|
-1.621
|
0.999
|
6.98
|
n-decane
|
0.894
|
-1.668
|
0.999
|
7.43
|
Pent-1-ene
|
0.754
|
-2.014
|
0.999
|
6.27
|
Hex-1-ene
|
0.844
|
-2.181
|
0.999
|
7.02
|
Hept-1-ene
|
0.845
|
-2.075
|
0.999
|
7.03
|
Oct-1-ene
|
0.855
|
-1.991
|
0.999
|
7.11
|
Non-1-ene
|
1.026
|
-2.388
|
0.999
|
8.53
|
Dec-1-ene
|
1.053
|
-2.338
|
0.999
|
8.76
|
Pent-1-yne
|
0.741
|
-1.979
|
0.999
|
6.16
|
Hex-1-yne
|
0.845
|
-2.183
|
0.999
|
7.03
|
Hept-1-yne
|
0.850
|
-2.087
|
0.999
|
7.07
|
Oct-1-yne
|
0.857
|
-1.998
|
0.999
|
7.13
|
Non-1-yne
|
1.028
|
-2.396
|
0.999
|
8.55
|
Dec-1-yne
|
1.093
|
-2.445
|
0.982
|
9.09
|
Cyclopentane
|
0.949
|
-2.741
|
0.999
|
7.89
|
Cyclohexane
|
0.925
|
-2.578
|
0.999
|
7.69
|
Cycloheptane
|
0.936
|
-2.533
|
0.999
|
7.78
|
Cyclooctane
|
0.933
|
-2.445
|
0.999
|
7.76
|
Cyclononane
|
0.882
|
-2.188
|
0.999
|
7.33
|
Methanol
|
1.989
|
-5.611
|
0.999
|
16.54
|
Ethanol
|
2.042
|
-5.718
|
0.999
|
16.98
|
Benzene
|
1.086
|
-3.860
|
0.999
|
9.03
|
Toluene
|
0.975
|
-3.352
|
0.999
|
8.11
|
Ethylbenzene
|
0.980
|
-3.154
|
0.999
|
8.15
|
Propylbenzene
|
0.878
|
-2.694
|
0.999
|
7.30
|
Acetone
|
1.294
|
-4.545
|
0.999
|
10.76
|
Butan-2-one
|
1.408
|
-4.853
|
0.999
|
11.71
|
|
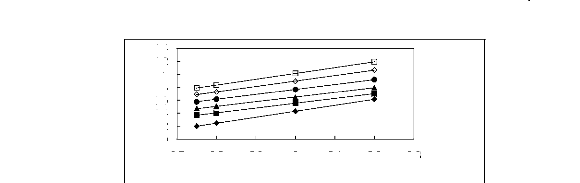
1.4
1.2
1
ln(111:i3)
0.8
0.6
0.4
0.2
0
2.7 2.8 2.9 3 3.1 3.2 3.3
1000K/T
Figure 5-17: Plots of versus for alkanes in
[3C6C14P] [PF6] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
n-pentane, () n-hexane,
(?) n-heptane, (?) n-octane, () n-nonane and (?)
n-decane.
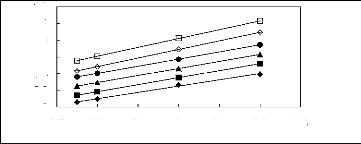
ln( IIP i3)
0.8
0.6
0.4
0.2
1.2
0
1
2.7 2.8 2.9 3 3.1 3.2 3.3
1000K/T
Figure 5-18: Plots of versus for alk-1-enes in
[3C6C14P] [PF6] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
pent-1-ene, () hex-1-ene,
(?) hept-1-ene, (?) oct-1-ene and () non-1-ene.
ln(111:13)
|
0.6
0.4
0.2
0 -0.2 -0.4 -0.6
|
|
|
2.7 2.8 2.9 3 3.1 3.2 3.3
1000K/T
Figure 5-19: Plots of versus for alk-1-ynes in
[3C6C14P] [PF6] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
pent-1-yne, () hex-1-yne,
(?) hept-1-yne and (?) oct-1-yne.
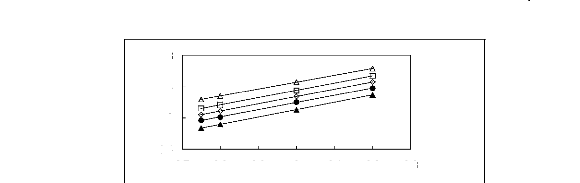
0.8
0.4
ln(I I 113)
0
-0.4
2.7 2.8 2.9 3 3.1 3.2 3.3
1000K/T
Figure 5-20: Plots of versus for cycloalkanes in
[3C6C14P] [PF6] together with a linear
correlation of the data using the Gibbs-Helmholtz equation;
(?) cyclopentane, (?) cyclohexane,
() cycloheptane, (?) cyclooctane and (?)
Cyclononane.
2.7 2.8 2.9 3 3.1 3.2 3.3
1000K/T
Figure 5-21: Plots of versus for alkanols in
[3C6C14P] [PF6] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
methanol and (?) ethanol
2.7 2.8 2.9 3 3.1 3.2 3.3
1000K/T
Figure 5-22: Plots of versus for alkylbenzenes
in [3C6C14P] [PF6] together with a
linear correlation of the data using the Gibbs-Helmholtz
equation; () benzene, () toluene,
(?) ethylbenzene and (?) propylbenzene.
2.7 2.8 2.9 3 3.1 3.2 3.3
1000K/T
Figure 5-23: Plots of versus for ketones in
[3C6C14P] [PF6] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
acetone and () butan-2-one.
ln(I 1 113)
|
1.2 0.9 0.6 0.3
0 -0.3 -0.6
|
|
|
0 1 2 3 4 5 6 7 8 9 10 11
Nc
Figure 5-24: Plots of versus the number of
carbon atoms at 313.15 K for ()n-alkanes, ()
alk-1-enes, (?) alk-1-ynes, (?) cycloalkanes, () alkanols, (?)
alkylbenzenes and (?)
ketones in [3C6C14P] [PF6].
5.1.5. Methyltrioctylammonium bis
(trifluoromethylsulfonyl) imide, [C13C8N] [Tf2N]. Table 5-14: Activity
coefficients at infinite dilution of organic solutes in
methyltrioctylammonium bis (trifluoromethylsulfonyl) imide
with n3 = 1.77 mmol (25.33 %) at
T = (303.15, 313.15 and
323.15) K.
|
Experimental at
|
/K
|
|
Solute
|
n3/mmol
|
T=303.15
|
T=313.15
|
T=323.15
|
n-pentane
|
1.77
|
1.47
|
1.44
|
1.42
|
n-hexane
|
1.77
|
1.70
|
1.72
|
1.69
|
n-heptane
|
1.77
|
1.85
|
1.83
|
1.83
|
n-octane
|
1.77
|
2.17
|
2.06
|
2.04
|
n-nonane
|
1.77
|
2.57
|
2.39
|
2.21
|
n-decane
|
1.77
|
3.20
|
2.72
|
2.50
|
Pent-1-ene
|
1.77
|
1.16
|
1.17
|
1.14
|
Hex-1-ene
|
1.77
|
1.22
|
1.21
|
1.21
|
Hept-1-ene
|
1.77
|
1.44
|
1.43
|
1.42
|
Oct-1-ene
|
1.77
|
1.69
|
1.67
|
1.66
|
Non-1-ene
|
1.77
|
1.99
|
1.91
|
1.85
|
Dec-1-ene
|
1.77
|
2.22
|
2.11
|
2.05
|
Pent-1-yne
|
1.77
|
0.72
|
0.74
|
0.75
|
Hex-1-yne
|
1.77
|
0.83
|
0.86
|
0.87
|
Hept-1-yne
|
1.77
|
0.89
|
0.91
|
0.92
|
Oct-1-yne
|
1.77
|
1.05
|
1.06
|
1.08
|
Non-1-yne
|
1.77
|
1.15
|
1.17
|
1.18
|
Dec-1-yne
|
1.77
|
1.30
|
1.31
|
1.34
|
Cyclopentane
|
1.77
|
0.90
|
0.98
|
0.97
|
Cyclohexane
|
1.77
|
1.18
|
1.19
|
1.16
|
Cycloheptane
|
1.77
|
1.32
|
1.31
|
1.26
|
Cyclooctane
|
1.77
|
1.54
|
1.47
|
1.43
|
Cyclononane
|
1.77
|
1.74
|
1.68
|
1.61
|
Methanol
|
1.77
|
1.26
|
1.19
|
1.11
|
Ethanol
|
1.77
|
1.32
|
1.24
|
1.13
|
Benzene
|
1.77
|
0.45
|
0.43
|
0.43
|
Toluene
|
1.77
|
0.51
|
0.52
|
0.53
|
Acetone
|
1.77
|
0.35
|
0.38
|
0.44
|
Butan-2-one
|
1.77
|
0.34
|
0.35
|
0.39
|
|
Table 5-15: Activity coefficients at infinite
dilution of organic solutes in
methyltrioctylammonium bis (trifluoromethylsulfonyl) imide
with n3 = 2.044 mmol (29.63 %)
at T = (303.15, 313.15 and
323.15) K.
Experimental at /K
Solute
|
n3/mmol
|
T=303.15
|
T=313.15
|
T=323.15
|
n-pentane
|
2.044
|
1.43
|
1.44
|
1.44
|
n-hexane
|
2.044
|
1.68
|
1.60
|
1.61
|
n-heptane
|
2.044
|
1.83
|
1.81
|
1.79
|
n-octane
|
2.044
|
2.23
|
2.16
|
2.10
|
n-nonane
|
2.044
|
2.59
|
2.39
|
2.21
|
n-decane
|
2.044
|
3.00
|
2.72
|
2.50
|
Pent-1-ene
|
2.044
|
1.14
|
1.11
|
1.12
|
Hex-1-ene
|
2.044
|
1.30
|
1.29
|
1.27
|
Hept-1-ene
|
2.044
|
1.44
|
1.43
|
1.42
|
Oct-1-ene
|
2.044
|
1.69
|
1.67
|
1.66
|
Non-1-ene
|
2.044
|
1.99
|
1.91
|
1.85
|
Dec-1-ene
|
2.044
|
2.22
|
2.11
|
2.05
|
Pent-1-yne
|
2.044
|
0.74
|
0.74
|
0.77
|
Hex-1-yne
|
2.044
|
0.83
|
0.84
|
0.85
|
Hept-1-yne
|
2.044
|
0.89
|
0.91
|
0.92
|
Oct-1-yne
|
2.044
|
1.05
|
1.06
|
1.08
|
Non-1-yne
|
2.044
|
1.15
|
1.17
|
1.18
|
Dec-1-yne
|
2.044
|
1.30
|
1.31
|
1.34
|
Cyclopentane
|
2.044
|
1.10
|
1.00
|
0.99
|
Cyclohexane
|
2.044
|
1.20
|
1.15
|
1.14
|
Cycloheptane
|
2.044
|
1.34
|
1.29
|
1.28
|
Cyclooctane
|
2.044
|
1.52
|
1.47
|
1.43
|
Cyclononane
|
2.044
|
1.74
|
1.68
|
1.61
|
Methanol
|
2.044
|
1.24
|
1.17
|
1.15
|
Ethanol
|
2.044
|
1.34
|
1.24
|
1.17
|
Benzene
|
2.044
|
0.43
|
0.45
|
0.47
|
Toluene
|
2.044
|
0.51
|
0.52
|
0.53
|
Acetone
|
2.044
|
0.35
|
0.38
|
0.44
|
Butan-2-one
|
2.044
|
0.34
|
0.37
|
0.41
|
|
Table 5-16: Average activity coefficients at
infinite dilution of organic solutes in
methyltrioctylammonium bis (trifluoromethylsulfonyl)
imide
at T = (303.15, 313.15 and 323.15) K.
Experimental
|
at /K
|
|
Solute
|
T=303.15
|
T=313.15
|
T=323.15
|
n-pentane
|
1.45
|
1.44
|
1.43
|
n-hexane
|
1.69
|
1.66
|
1.65
|
n-heptane
|
1.84
|
1.82
|
1.81
|
n-octane
|
2.20
|
2.11
|
2.07
|
n-nonane
|
2.58
|
2.39
|
2.21
|
n-decane
|
3.10
|
2.72
|
2.50
|
Pent-1-ene
|
1.15
|
1.14
|
1.13
|
Hex-1-ene
|
1.26
|
1.25
|
1.24
|
Hept-1-ene
|
1.44
|
1.43
|
1.42
|
Oct-1-ene
|
1.69
|
1.67
|
1.66
|
Non-1-ene
|
1.99
|
1.91
|
1.85
|
Dec-1-ene
|
2.22
|
2.11
|
2.05
|
Pent-1-yne
|
0.73
|
0.74
|
0.76
|
Hex-1-yne
|
0.83
|
0.85
|
0.86
|
Hept-1-yne
|
0.89
|
0.91
|
0.92
|
Oct-1-yne
|
1.05
|
1.06
|
1.08
|
Non-1-yne
|
1.15
|
1.17
|
1.18
|
Dec-1-yne
|
1.30
|
1.31
|
1.34
|
Cyclopentane
|
1.00
|
0.99
|
0.98
|
Cyclohexane
|
1.19
|
1.17
|
1.15
|
Cycloheptane
|
1.33
|
1.30
|
1.27
|
Cyclooctane
|
1.53
|
1.47
|
1.43
|
Cyclononane
|
1.74
|
1.68
|
1.61
|
Methanol
|
1.25
|
1.18
|
1.13
|
Ethanol
|
1.33
|
1.24
|
1.15
|
Benzene
|
0.44
|
0.44
|
0.45
|
Toluene
|
0.51
|
0.52
|
0.53
|
Acetone
|
0.35
|
0.38
|
0.44
|
Butan-2-one
|
0.34
|
0.36
|
0.40
|
|
Table 5-17: Excess molar enthalpies at
infinite dilution of organic solutes in the ionic
liquid methyltrioctylammonium bis (trifluoromethylsulfonyl)
imide, calculated using the Gibbs-
Helmholtz equation.
SOLUTE
|
Linear regression using Eq.(2-11)
|
|
|
|
|
n-pentane
|
0.694
|
0.142
|
1.00
|
5.77
|
n-hexane
|
0.120
|
0.128
|
0.92
|
1.00
|
n-heptane
|
0.082
|
0.338
|
0.97
|
0.68
|
n-octane
|
0.305
|
-0.220
|
0.96
|
2.53
|
n-nonane
|
0.774
|
-1.606
|
1.00
|
6.44
|
n-decane
|
1.075
|
-2.425
|
0.99
|
8.93
|
Pent-1-ene
|
0.088
|
-0.150
|
1.00
|
0.73
|
Hex-1-ene
|
0.080
|
-0.033
|
1.00
|
0.67
|
Hept-1-ene
|
0.105
|
0.028
|
0.96
|
0.87
|
Oct-1-ene
|
0.120
|
0.128
|
0.92
|
1.00
|
Non-1-ene
|
0.419
|
-0.698
|
0.98
|
3.48
|
Dec-1-ene
|
0.419
|
-0.580
|
0.97
|
3.48
|
Pent-1-yne
|
-0.201
|
0.348
|
0.97
|
-1.67
|
Hex-1-yne
|
-0.178
|
0.402
|
0.96
|
-1.48
|
Hept-1-yne
|
-0.111
|
0.250
|
1.00
|
-0.92
|
Oct-1-yne
|
-0.140
|
0.517
|
0.97
|
-1.16
|
Non-1-yne
|
-0.129
|
0.563
|
0.97
|
-1.07
|
Dec-1-yne
|
-0.114
|
0.640
|
0.96
|
-0.95
|
Cyclopentane
|
0.101
|
-3.333
|
1.00
|
0.84
|
Cyclohexane
|
0.171
|
-0.390
|
1.00
|
1.42
|
Cycloheptane
|
0.231
|
-0.476
|
1.00
|
1.92
|
Cyclooctane
|
0.338
|
-0.692
|
0.99
|
2.81
|
Cyclononane
|
0.386
|
-0.715
|
1.00
|
3.21
|
Methanol
|
0.505
|
-1.445
|
0.99
|
4.20
|
Ethanol
|
0.727
|
-2.113
|
1.00
|
6.05
|
Benzene
|
-0.112
|
-0.454
|
0.75
|
-0.93
|
Toluene
|
-0.286
|
0.267
|
0.97
|
-2.38
|
Acetone
|
-0.962
|
2.062
|
0.98
|
-8.00
|
Butan-2-one
|
-1.029
|
2.349
|
1.00
|
-8.56
|
|
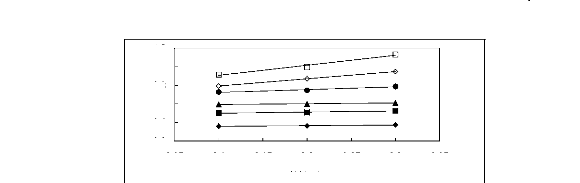
3.05 3.1 3.15 3.2 3.25 3.3 3.35
1000K/T
1.2
1
1n(L13)
0.8
0.6
0.4
0.2
Figure 5-25: Plots of versus for alkanes in
[C13C8N] [Tf2N] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
n-pentane, () n-hexane,
(?) n-heptane, (?) n-octane, () n-nonane and (?)
n-decane.
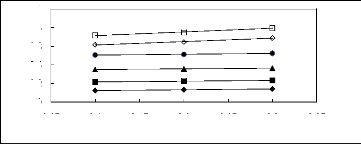
3.05 3.1 3.15 3.2 3.25 3.3 3.35
1000/T/K-1
In(L13)
0.8
0.6
0.4
0.2
0
1
Figure 5-26: Plots of versus for alk-1-enes in
[C13C8N] [Tf2N] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
pent-1-ene, () hex-1-ene,
(?) hept-1-ene, (?) oct-1-ene, () non-1-ene and
(?) dec-1-ene.
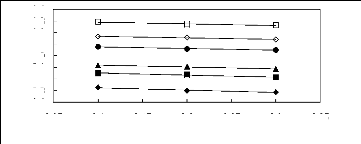
3.05 3.1 3.15 3.2 3.25 3.3 3.35
1000K/T
In(L13)
-0.1
-0.2
-0.3
-0.4
0.4
0.3
0.2
0.1
0
Figure 5-27: Plots of versus for alk-1-ynes in
[C13C8N] [Tf2N] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
pent-1-y-ne, () hex-1-yne,
(?) hept-1-yne, (?) oct-1-yne, () non-1-yne and
(?) dec-1-yne.
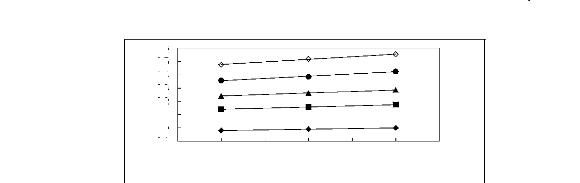
3.05 3.1 3.15 3.2 3.25 3.3 3.35
1000K/T
0.6
0.5
0.4
lii(LP13)
0.3
0.2
0.1
0
-0.1
Figure 5-28: Plots of versus for cycloalkanes in
[C13C8N] [Tf2N] together with a
linear correlation of the data using the Gibbs-Helmholtz
equation; () cyclopentane,
() cyclohexane, (?) cycloheptane, (?) cyclooctane
and () cyclononane.
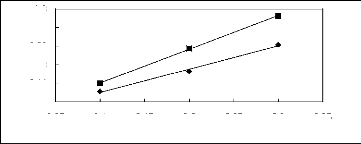
3.05 3.1 3.15 3.2 3.25 3.3 3.35
1000K/T
lii(LP13)
0.26
0.22
0.18
0.14
0.3
0.1
Figure 5-29: Plots of versus for alkanols in
[C13C8N] [Tf2N] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
methanol and () ethanol.
|
-0.6 -0.65 -0.7 -0.75
|
|
|
ln(ffi3)
|
|
|
|
-0.8
-0.85
|
|
|
|
|
|
|
3.05 3.1 3.15 3.2 3.25 3.3 3.35
1000K/T
Figure 5-30: Plots of versus for alkylbenzenes
in [C13C8N] [Tf2N] together with a
linear correlation of the data using the Gibbs-Helmholtz
equation; () benzene and () toluene.
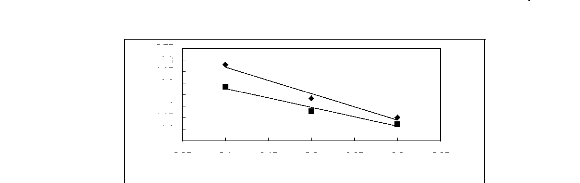
-0.75
-0.8
-0.85
hi( Du 13)
-0.9
-0.95
-1
-1.05
-1.1
-1.15
3.05 3.1 3.15 3.2 3.25 3.3 3.35
1000K/T
Figure 5-31: Plots of versus for ketones in
[C13C8N] [Tf2N] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
acetone and () butan-2-one.
0 1 2 3 4 5 6 7 8 9 10 11
Number of Carbon atoms, Nc
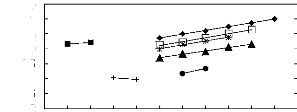
ln( LP13)
-0.5
-1.5
0.5
1.5
-1
-2
0
1
Figure 5-32: Plots of versus the number of
carbon atoms at 313.15 K for () n-alkanes,
(?) alk-1-enes, and (?) alk-1-ynes, (*) cycloalkanes, ()
alkanols, and (?) alkylbenzenes and
(+) ketones in [C13C8N] [Tf2N].
| 

