CHAPTER FIVE: RESULTS
Chapter overview
Results presented in this work consist of the following:
· Infinite dilution activity coefficients data for various
organic solutes in n-hexadecane and seven different FILs, obtained from
Gas-Liquid Chromatography;
· Excess molar enthalpies at infinite dilution of the same
solutes in the fluorinated ionic liquids, calculated from experimental infinite
dilution activity coefficients data;
· Correlation of experimental infinite dilution activity
coefficient data with temperature and the carbon chain length of solutes;
· Infinite dilution activity coefficients data for six
organic solutes in NMP and one fluorinated ionic liquid;
· Limiting selectivities and capacities of the seven
investigated fluorinated ionic liquids for selected separation problems;
· Estimation of experimental errors in determining activity
coefficients, Excess molar enthalpies, selectivities and capacities at infinite
dilution.
Equations (3-10) and (3-90) were used to calculate IDACs from
experimental parameters obtained by the GC method and the IGST respectively.
Excess molar enthalpies at infinite dilution were determined using equation
(2-11). Some experimental data obtained in this study have been published as
part of collaborative work with Prof. Nirmala Deenadayalu ([C13C8] [Tf2N]) and
Mr. Eugene Olivier ([EMIM] [TfO], [BMIM] [SbF6] and [MOIM] [PF6]). These
results are also included in this chapter.
5.1. Results from Gas-Liquid Chromatography
5.1.1. Hexadecane
Table 5-1: Infinite dilution activity
coefficients of selected organic solutes in n-hexadecane.
Solutes
|
T /K
|
|
|
R.D#./ %
|
n-pentane n-pentane n-hexane n-hexane n-hexane n-heptane
n-heptane
|
323.15 333.15 303.15 313.15 323.15 303.15
? )
1
323.15
lit
|
0.839 0.870 0.882 0.892 0.896 0.882 0.890
|
0.814a
0.833
a
0.880-0.904bc
0.870-0.910
b
0.860-0.905 a bd
0.916-0.927 b
0.867-0.921
ad
|
3.1 4.4 1.8 1.4 0.6 4.3 0.4
|
Hex-1-ene
|
323.15
|
0.862
|
0.855 a
|
0.8
|
Hept-1-ene
|
323.15
|
0.866
|
0.902 a
|
4.0
|
Cyclopentane
|
323.15
|
0.695
|
0.686 a
|
1.3
|
Cyclohexane
|
303.15
|
0.775
|
0.795 b
|
2.5
|
Cyclohexane
|
313.15
|
0.776
|
0.778 b
|
0.3
|
Cyclohexane
|
323.15
|
0.736
|
0.739-0.787 ad
|
3.5
|
Benzene
|
303.15
|
1.071
|
1.105-1.110 be
|
3.3
|
Benzene
|
313.15
|
1.010
|
1.006-1.051 b
|
0.4
|
Benzene
|
323.15
|
0.953
|
0.932-0.995 ad
|
2.1
|
Toluene
|
323.15
|
0.953
|
0.941 a
|
1.3
|
Dichloromethane
|
303.15
|
1.428
|
1.440b
|
0.8
|
|
a Schult et al. (2001); bChien et al
(1981) c Cruickshank et al. (1966b); d Castells et al.
(1990);
e Gainey and Young (1968)
# Relative deviation, R.D., given by
5.1.2. Trihexyltetradecylphosphonium bis
(trifluoromethylsulfonyl) imide,
[3C6C14P] [Tf2N]
Table 5-2: Activity coefficients at infinite
dilution of organic solutes in
trihexyltetradecylphosphonium bis (trifluoromethylsulfonyl)
imide with solvent column loading
n3 = 1.577 mmol (29.5 %) at T = (313.15,
333.15,
|
353.15 and 373.15) K.
|
|
Experimental
|
at /K
|
|
|
Solute
|
n3/ mmol
|
T=313.15
|
T=333.15
|
T=353.15
|
T=373.15
|
n-pentane
|
1.577
|
0.970
|
0.976
|
0.988
|
0.990
|
n-hexane
|
1.577
|
1.101
|
1.119
|
1.126
|
1.130
|
n-heptane
|
1.577
|
1.163
|
1.249
|
1.315
|
1.434
|
n-octane
|
1.577
|
1.405
|
1.443
|
1.459
|
1.495
|
n-nonane
|
1.577
|
1.607
|
1.681
|
1.769
|
1.871
|
Pent-1-ene
|
1.577
|
0.810
|
0.833
|
0.841
|
0.862
|
Hex-1-ene
|
1.577
|
0.909
|
0.932
|
0.941
|
0.943
|
Hept-1-ene
|
1.577
|
1.030
|
1.064
|
1.102
|
1.123
|
Oct-1-ene
|
1.577
|
1.163
|
1.384
|
1.610
|
1.905
|
Pent-1-yne
|
1.577
|
0.611
|
0.640
|
0.662
|
0.701
|
Hex-1-yne
|
1.577
|
0.691
|
0.707
|
0.718
|
0.730
|
Hept-1-yne
|
1.577
|
0.730
|
0.747
|
0.764
|
0.780
|
Oct-1-yne
|
1.577
|
0.841
|
0.872
|
0.890
|
0.899
|
Nony-1-ne
|
1.577
|
0.850
|
0.912
|
0.954
|
0.977
|
Cyclopentane
|
1.577
|
0.686
|
0.696
|
0.714
|
0.723
|
Cyclohexane
|
1.577
|
0.797
|
0.800
|
0.802
|
0.805
|
Cycloheptane
|
1.577
|
0.865
|
0.882
|
0.895
|
0.910
|
Cyclooctane
|
1.577
|
0.965
|
1.000
|
1.065
|
1.119
|
Methanol
|
1.577
|
1.077
|
1.025
|
0.921
|
0.865
|
Ethanol
|
1.577
|
1.270
|
1.096
|
0.953
|
0.880
|
Propan-1-ol
|
1.577
|
1.279
|
1.155
|
1.070
|
0.954
|
Butan-1-ol
|
1.577
|
1.425
|
1.194
|
1.090
|
0.949
|
Benzene
|
1.577
|
0.390
|
0.403
|
0.414
|
0.432
|
Toluene
|
1.577
|
0.450
|
0.471
|
0.485
|
0.512
|
Acetone
|
1.577
|
0.299
|
0.318
|
0.328
|
0.332
|
Butan-2-one
|
1.577
|
0.317
|
0.329
|
0.331
|
0.333
|
|
Table 5-3: Activity coefficients at infinite
dilution of organic solutes in
trihexyltetradecylphosphonium bis (trifluoromethylsulfonyl)
imide with solvent column loading
n3 =2.236 mmol (31.7 %) at T = (313.15,
333.15,
|
353.15 and 373.15) K.
|
|
Experimental
|
at /K
|
|
|
Solute
|
n3/ mmol
|
T=313.15
|
T=333.15
|
T=353.15
|
T=373.15
|
n-pentane
|
2.236
|
0.950
|
0.980
|
0.992
|
1.014
|
n-hexane
|
2.236
|
1.091
|
1.103
|
1.122
|
1.130
|
n-heptane
|
2.236
|
1.139
|
1.257
|
1.309
|
1.412
|
n-octane
|
2.236
|
1.403
|
1.439
|
1.463
|
1.491
|
n-nonane
|
2.236
|
1.593
|
1.659
|
1.777
|
1.867
|
Pent-1-ene
|
2.236
|
0.802
|
0.815
|
0.843
|
0.838
|
Hex-1-ene
|
2.236
|
0.905
|
0.910
|
0.919
|
0.941
|
Hept-1-ene
|
2.236
|
1.034
|
1.066
|
1.100
|
1.121
|
Oct-1-ene
|
2.236
|
1.159
|
1.382
|
1.606
|
1.901
|
Pent-1-yne
|
2.236
|
0.615
|
0.632
|
0.664
|
0.697
|
Hex-1-yne
|
2.236
|
0.695
|
0.711
|
0.722
|
0.734
|
Hept-1-yne
|
2.236
|
0.722
|
0.751
|
0.760
|
0.784
|
Oct-1-yne
|
2.236
|
0.831
|
0.858
|
0.882
|
0.899
|
Nony-1-ne
|
2.236
|
0.856
|
0.918
|
0.958
|
0.981
|
Cyclopentane
|
2.236
|
0.688
|
0.700
|
0.712
|
0.719
|
Cyclohexane
|
2.236
|
0.793
|
0.794
|
0.794
|
0.795
|
Cycloheptane
|
2.236
|
0.875
|
0.880
|
0.887
|
0.900
|
Cyclooctane
|
2.236
|
0.961
|
0.996
|
1.073
|
1.121
|
Methanol
|
2.236
|
1.089
|
1.027
|
0.923
|
0.861
|
Ethanol
|
2.236
|
1.268
|
1.094
|
0.949
|
0.884
|
Propan-1-ol
|
2.236
|
1.281
|
1.149
|
1.074
|
0.952
|
Butan-1-ol
|
2.236
|
1.421
|
1.188
|
1.080
|
0.959
|
Benzene
|
2.236
|
0.392
|
0.407
|
0.420
|
0.442
|
Toluene
|
2.236
|
0.454
|
0.473
|
0.487
|
0.508
|
Acetone
|
2.236
|
0.295
|
0.300
|
0.320
|
0.330
|
Butan-2-one
|
2.236
|
0.325
|
0.326
|
0.333
|
0.335
|
|
Table 5-4: Average activity coefficients at
infinite dilution of organic solutes in
trihexyltetradecylphosphonium bis (trifluoromethylsulfonyl)
imide at T = (313.15, 333.15,
353.15 and 373.15) K.
Experimental at /K
Solute
|
T=313.15
|
T=333.15
|
T=353.15
|
T=373.15
|
n-pentane
|
0.960
|
0.978
|
0.990
|
1.002
|
n-hexane
|
1.096
|
1.111
|
1.124
|
1.130
|
n-heptane
|
1.151
|
1.253
|
1.312
|
1.423
|
n-octane
|
1.404
|
1.441
|
1.461
|
1.493
|
n-nonane
|
1.600
|
1.670
|
1.773
|
1.869
|
Pent-1-ene
|
0.806
|
0.824
|
0.842
|
0.850
|
Hex-1-ene
|
0.907
|
0.921
|
0.930
|
0.942
|
Hept-1-ene
|
1.032
|
1.065
|
1.101
|
1.122
|
Oct-1-ene
|
1.161
|
1.383
|
1.608
|
1.903
|
Pent-1-yne
|
0.613
|
0.636
|
0.663
|
0.699
|
Hex-1-yne
|
0.693
|
0.709
|
0.720
|
0.732
|
Hept-1-yne
|
0.726
|
0.749
|
0.762
|
0.782
|
Oct-1-yne
|
0.836
|
0.865
|
0.886
|
0.899
|
Non-1-yne
|
0.853
|
0.915
|
0.956
|
0.979
|
Cyclopentane
|
0.687
|
0.698
|
0.713
|
0.721
|
Cyclohexane
|
0.795
|
0.797
|
0.798
|
0.800
|
Cycloheptane
|
0.870
|
0.881
|
0.891
|
0.905
|
Cyclooctane
|
0.963
|
0.998
|
1.069
|
1.120
|
Methanol
|
1.083
|
1.026
|
0.922
|
0.863
|
Ethanol
|
1.269
|
1.095
|
0.951
|
0.882
|
Propan-1-ol
|
1.280
|
1.152
|
1.072
|
0.953
|
Butan-1-ol
|
1.423
|
1.191
|
1.085
|
0.954
|
Benzene
|
0.391
|
0.405
|
0.417
|
0.437
|
Toluene
|
0.452
|
0.472
|
0.486
|
0.510
|
Acetone
|
0.297
|
0.327
|
0.332
|
0.334
|
Butan-2-one
|
0.321
|
0.328
|
0.324
|
0.331
|
|
Table 5-5: Partial molar excess enthalpies at
infinite dilution for organic solutes in the
ionic liquid trihexyltetradecylphosphonium bis
(trifluoromethylsulfonyl) imide, calculated from
the Gibbs-Helmholtz
equation.
SOLUTE
|
Linear regression using Eq.(2-11)
|
|
|
|
|
n-pentane
|
-0.083
|
0.223
|
0.995
|
-0.69
|
n-hexane
|
-0.061
|
0.286
|
0.982
|
-0.51
|
n-heptane
|
-0.398
|
1.413
|
0.987
|
-3.31
|
n-octane
|
-0.116
|
0.710
|
0.990
|
-0.96
|
n-nonane
|
-0.306
|
1.440
|
0.988
|
-2.54
|
Pent-1-ene
|
-0.107
|
0.126
|
0.985
|
-0.89
|
Hex-1-ene
|
-0.072
|
0.132
|
0.995
|
-0.60
|
Hept-1-ene
|
-0.166
|
0.563
|
0.994
|
-1.38
|
Oct-1-ene
|
-0.953
|
3.186
|
0.997
|
-7.92
|
Pent-1-yne
|
-0.253
|
0.312
|
0.981
|
-2.10
|
Hex-1-yne
|
-0.105
|
-0.029
|
0.997
|
-0.87
|
Hept-1-yne
|
-0.140
|
0.129
|
0.991
|
-1.17
|
Oct-1-yne
|
-0.142
|
0.278
|
0.984
|
-1.18
|
Nony-1-ne
|
-0.270
|
0.710
|
0.970
|
-2.24
|
Cyclopentane
|
-0.097
|
-0.065
|
0.990
|
-0.81
|
Cyclohexane
|
-0.011
|
-0.192
|
0.982
|
-0.10
|
Cycloheptane
|
-0.075
|
0.100
|
0.987
|
-0.63
|
Cyclooctane
|
-0.303
|
0.923
|
0.978
|
-2.52
|
Methanol
|
0.459
|
-1.372
|
0.978
|
3.81
|
Ethanol
|
0.723
|
-2.078
|
0.991
|
6.02
|
Propan-1-ol
|
0.557
|
-1.528
|
0.987
|
4.63
|
Butan-1-ol
|
0.757
|
-2.075
|
0.990
|
6.30
|
Benzene
|
-0.211
|
-0.269
|
0.980
|
-1.75
|
Toluene
|
-0.228
|
-0.067
|
0.986
|
-1.90
|
Acetone
|
-0.079
|
-0.880
|
0.994
|
-0.66
|
Butan-2-one
|
-0.218
|
-0.514
|
0.998
|
-1.81
|
|
0.8
0.6
ln(1113)
0.4
0.2
0
-0.2
2.6 2.7 2.8 2.9 3 3.1 3.2 3.3
1000K/T
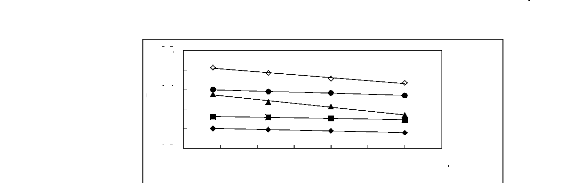
Figure 5-1: Plots of versus for alkanes in
[3C6C14P] [Tf2N] together with a
linear correlation of the data using the Gibbs-Helmholtz
equation; () n-pentane,
() n-hexane, (?) n-heptane and (?) n-octane, ()
n-nonane.
2.6 2.7 2.8 2.9 3 3.1 3.2 3.3
1000K/T
ln(1313)
-0.2
-0.4
0.8
0.6
0.4
0.2
0.0
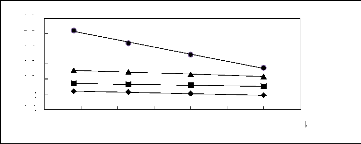
Figure 5-2: Plots of versus for alk-1-enes in
[3C6C14P] [Tf2N] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
pent-1-ene, () hex-1-ene,
(?) hept-1-ene and (?) oct-1-ene.
ln(1313)
-0.1
-0.2
-0.3
-0.4
-0.5
0.2
0.1
0
2.6 2.8 3 3.2 3.4
1000K/T
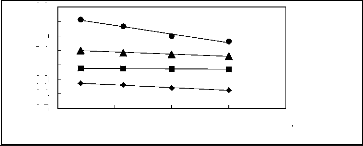
Figure 5-3: Plots of versus for cycloalkanes in
[3C6C14P] [Tf2N] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
cyclopentane, () cyclohexane,
(?) cycloheptane and (?) Cyclooctane.
0.0
-0.1
-0.2
ln( LP 13)
-0.3
-0.4
-0.5
-0.6
2.6 2.8 3 3.2 3.4
1000K/T
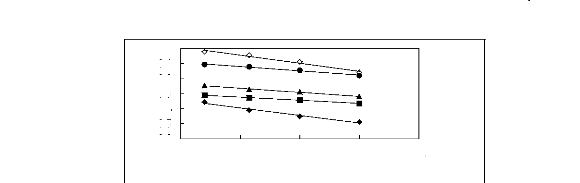
Figure 5-4: Plots of versus for alk-1-ynes in
[3C6C14P] [Tf2N] together with a linear
correlation of the data using the Gibbs-Helmholtz equation;
()pent-1-yne, () hex-1-yne,
(?) hept-1-yne, (?) oct-1-yne and ()
n-nonyne.
lii( LP 13)
|
0.4 0.3 0.2 0.1
0 -0.1 -0.2
|
|
|
2.6 2.7 2.8 2.9 3 3.1 3.2 3.3
1000K/T
Figure 5-5: Plots of versus for alkanols in
[3C6C14P] [Tf2N] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
methanol, () ethanol,
(?) propan-1-ol and (?) butan-1-ol.
ln( LP13)
|
-0.5 -0.6 -0.7 -0.8 -0.9
-1
|
|
|
2.6 2.8 3 3.2 3.4
1000K/T
Figure 5-6: Plots of versus for alkylbenzenes in
[3C6C14P] [Tf2N] together with a
linear correlation of the data using the Gibbs-Helmholtz
equation; () benzene and () toluene.
1n(L13)
|
-1.08 -1.12 -1.16 -1.20 -1.24
|
|
|
2.6 2.7 2.8 2.9 3 3.1 3.2 3.3
1000K/T
Figure 5-7: Plots of versus for ketones in
[3C6C14P] [Tf2N] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
acetone and () butan-2-one.
0 1 2 3 4 5 6 7 8 9 10
Number of Carbon atoms, Nc
Figure 5-8: Plots of versus the carbon number of
the solute at 313.15 K for () n-alkanes,
() alk-1-enes, (?) cycloalkanes, (?) alk-1-ynes () ketones,
(?) alkanols and (?)
alkylbenzenes in [3C6C14P] [Tf2N].
| 


