APPENDIX B: STRUCTURE OF IONIC LIQUIDS
[3C6C14P]+ [Tf2N] - [PF6]
[BF4]- [OMIM] + [SbF6]
[BMIM]+ [EMIM] + [TfO] - [C83C1N] +
Figure B-1: Ions present in the structure of
ionic liquids used in this work.
Appendix C: Origin and purity of chemicals
APPENDIX C: ORIGIN AND PURITY OF CHEMICALS
Table C-1: Origin and Stated purity of solutes
and solvents.
|
Compound
|
Origin
|
Stated purity (Mass %)
|
|
[3C6C14P] [Tf2N]
|
Cytec
|
= 0.98
|
|
[3C6C14P] [BF4]
|
Cytec
|
= 0.98
|
|
[3C6C14P] [PF6]
|
Cytec
|
= 0.98
|
|
[3C8C1N] [Tf2N]
|
Solvent innovation
|
0.98
|
|
[BMIM] [SbF6]
|
Capital Lab
|
= 0.98
|
|
[EMIM] TfO]
|
Capital Lab
|
= 0.98
|
|
[MOIM] [PF6]
|
Capital Lab
|
= 0.95
|
|
NMP
|
Merck
|
= 0.98
|
|
n-hexadecane
|
Acros
|
0.99
|
|
n-alkanes (C5 to C12)
|
Capital Lab
|
= 0.98
|
|
Alk-1-enes (C5 to C12)
|
Capital Lab
|
= 0.98
|
|
Alk-1-ynes (C5 to C12)
|
Capital Lab
|
= 0.98
|
|
Cycloalkanes (C5 to C10)
|
Capital Lab
|
= 0.98
|
|
n-alkanols (C1 to C4)
|
Capital Lab
|
= 0.98
|
|
Alkylbenzenes (C6 to C9)
|
Capital Lab
|
= 0.98
|
|
Ket-2-ones (C3 to C4)
|
Capital Lab
|
= 0.98
|
Table C-2: Densities of solvents after
purification at different temperatures-Accuracy: #177; 0.4
%.;*Interpolated
value;# Extrapolated data aFrom Rodriguez and Brennecke
(2006); b From
Pereiro et al. (2007); c From Kneisl
and Zondlo, (1987); d From Khasanshin et al (2009).
|
Solvents
|
293.15 K
|
303.15 K
|
313.15 K
|
323.15 K
|
333.15 K
|
Literature Data at
293.15 K
|
|
[3C6C14P] [Tf2N]
|
1.068870
|
1.062387
|
1.056497
|
1.051422
|
1.047132
|
|
|
[3C6C14P] [BF4]
|
-
|
-
|
-
|
0.925100
|
-
|
|
|
[3C6C14P] [PF6]
|
-
|
-
|
-
|
0.987150
|
-
|
|
|
[3C8C1N] [Tf2N]
|
1.112700
|
-
|
-
|
-
|
-
|
|
|
[BMIM] [SbF6]
|
1.694300
|
-
|
-
|
-
|
-
|
|
|
[EMIM] TfO]
|
1.387070
|
1.375511
|
1.369032
|
1.363705
|
1.359564
|
1.387392a*
|
|
[MOIM] [PF6]
|
1.238510
|
-
|
-
|
-
|
-
|
1.23957b
|
|
NMP
|
-
|
1.025173
|
1.017633
|
1.010888
|
1.004919
|
1.032313c#
|
|
n-hexadecane
|
0.770522
|
-
|
-
|
-
|
-
|
0.77418d#
|
Table C-3: Refractive indices of solvents
after purification at 293.15 K. #Extrapolated
data;
aMehra, (2003); bFrom
www.haochem.com?; cFrom
Pereiro et al. (2007).
|
Solvents
|
Refractive index
|
Literature Data
|
|
[3C6C14P] [Tf2N]
|
1.45069
|
|
|
[3C8C1N] [Tf2N]
|
-
|
|
|
[BMIM] [SbF6]
|
1.41568
|
|
|
[EMIM] TfO]
|
1.43434
|
|
|
[MOIM] [PF6]
|
1.42430
|
1.42440c
|
|
NMP
|
1.47047
|
1.465-1.470b
|
|
n-Hexadecane
|
1.43463
|
1.4356a#
|
Appendix D: Fugacities, critical data and ionization
energies
APPENDIX D: FUGACITIES, CRITICAL DATA AND
IONIZATION
ENERGIES
Table D-1: Saturation fugacity coefficients of
selected solutes at different temperatures
determined from second virial
coefficients (Smith et al (2005).
|
Solutes
|
303.15 K
|
313.15 K
|
323.15 K
|
|
n-hexane
|
0.982
|
0.977
|
0.970
|
|
Hex-1-ene
|
0.981
|
0.974
|
0.967
|
|
Cyclohexane
|
0.988
|
0.983
|
0.978
|
|
Methanol
|
0.997
|
0.995
|
0.993
|
|
Benzene
|
0.989
|
0.986
|
0.981
|
|
Acetone
|
0.988
|
0.984
|
0.980
|
Table D-2: Critical volumes, critical
temperatures,and ionization energies, IC of the
solutes and the carrier gas used in the calculation of the
virial coefficients. (Reference: CRC
Handbook of Chemistry and Physics).
|
Solute
|
TC /K
|
VC/
cm3.mol-1
|
IC/ kJ.mol-1
|
|
n-pentane
|
469.7
|
304
|
998.62
|
|
n-hexane
|
507.4
|
370
|
977.39
|
|
n-heptane
|
540.3
|
432
|
957.13
|
|
n-octane
|
568.8
|
492
|
947.48
|
|
n-nonane
|
594.7
|
555.2
|
937.83
|
|
Pent-1-ene
|
464.7
|
300
|
917.57
|
|
Hex-1-ene
|
504
|
350
|
910.82
|
|
Hept-1-ene
|
537.2
|
405
|
910.82
|
|
Oct-1-ene
|
566.6
|
464
|
909.85
|
|
Pent-1-yne
|
493.4
|
278
|
969.67
|
|
Hex-1-yne
|
539.29
|
331
|
960.02
|
|
Hept-1-yne
|
551.621
|
376.53
|
960.02
|
|
Oct-1-yne
|
598.46
|
441
|
960.02
|
|
Nony-1-ne
|
611
|
513.3
|
955.20
|
|
Cyclopentane
|
511.7
|
259
|
1014.05
|
|
Cyclohexane
|
553.8
|
308
|
951.34
|
|
Cycloheptane
|
604.2
|
353
|
961.95
|
|
Cyclooctane
|
647.2
|
410
|
941.69
|
|
Methanol
|
512.6
|
118
|
1046.86
|
|
Ethanol
|
516.2
|
167
|
1010.20
|
|
Propan-1-ol
|
536.7
|
218.5
|
986.07
|
|
Butan-1-ol
|
562.9
|
274
|
970.64
|
|
Benzene
|
562.1
|
259
|
892.10
|
|
Toluene
|
591.7
|
316
|
851.00
|
|
Acetone
|
508.1
|
209
|
935.90
|
|
Butan-2-one
|
535.6
|
267
|
918.54
|
|
Helium
|
5.2
|
57.5
|
2372.56
|
Appendix E: Calibration data
APPENDIX E: CALIBRATION DATA
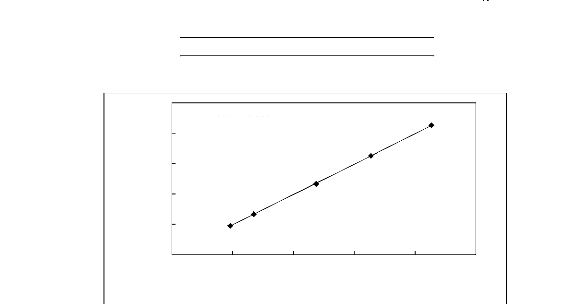
y = 1.001x - 0.229
R2 = 0.999
20 30 40 50 60 70
Display Temperature, (oC)
70
Actual Temperature, (°C)
60
50
40
30
20
Figure E-1: Temperature calibration curve for
the dilutor cell Pt 100.
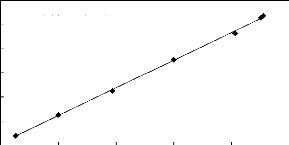
y = 1.149x + 3.572
R2 = 0.999
84 86 88 90 92 94
Display Pressure (kPa)
112
Actual Temperature (kPa)
110
108
106
104
102
100
Figure E-2: Pressure calibration curve for the
dilutor cell pressure transducer.
Appendix F: Selectivities and capacities
APPENDIX F: SELECTIVITIES AND CAPACITIES
Table F-1: Infinite dilution selectivity and
capacity data at 313.15 K for FIL`s and selected industrial solvents
investigated in the literature (Subscripts are references given in Chapter 6)
as well as in this work*; #Data obtained at 298.15
K.
Butan-2-one (2)
Benzene (2)
Hex-1-ene (2)
Acetone (2)
Hexane (1)
/benzene (2)
Cyclohexale
(1 )/benzene (2)
Methanol (1) /benzene (2)
Benzene (1)
/butan-2-one (2)
Hexane (1)
/he(-1-ene (2)
Methanol (1) /acetone (2)
Ethanol (1)
/butan-2-one (2)
Limiting Selectivity Limiting Capacity
|
[EMIM][BF4][1][2]
|
38.65
|
20.06
|
0.17
|
1.54
|
2.02
|
0.39
|
0.47
|
0.41
|
0.02
|
0.91
|
0.62
|
|
[EMIM] [Tf2N] [3][4][5]
|
21.12
|
9.75
|
1.08
|
2.40
|
2.08
|
3.17
|
3.54
|
0.83
|
0.08
|
2.44
|
2.00
|
|
[MMIM] [Tf2N] [4]
|
27.26
|
15.48
|
-
|
-
|
2.24
|
-
|
-
|
0.74
|
0.06
|
-
|
-
|
|
[BMIM][BF4][2][6][7]
|
23.84
|
12.53
|
0.52
|
1.51
|
1.70
|
1.23
|
0.87
|
0.41
|
0.03
|
0.98
|
0.63
|
|
[BMIM] [Tf2N] [4][8]
|
15.13
|
9.16
|
1.23
|
-
|
1.89
|
2.97
|
-
|
1.12
|
0.14
|
2.70
|
-
|
|
[BMIM][TfO][9]
|
23.04
|
10.66
|
0.44
|
-
|
2.23
|
0.79
|
-
|
0.63
|
0.06
|
1.13
|
-
|
|
[DMPIM][BF4][10][11]
|
70.40
|
31.77
|
0.24
|
-
|
-
|
0.34
|
-
|
0.28
|
-
|
0.40
|
-
|
|
[EDMIM] [Tf2N] [3]
|
23.03
|
13.22
|
1.33
|
1.92
|
2.07
|
3.56
|
3.58
|
0.91
|
0.08
|
2.44
|
1.75
|
|
[HMIM][BF4][2][12]
|
19.53
|
5.61
|
0.41
|
1.49
|
2.00
|
0.82
|
1.42
|
0.61
|
0.11
|
1.22
|
0.91
|
|
[HMIM][PF6][13]
|
18.46
|
10.19
|
1.54
|
-
|
2.11
|
-
|
-
|
0.96
|
0.11
|
-
|
-
|
|
[HMIM] [Tf2N] [14][15][16]
|
11.22
|
6.70
|
1.65
|
1.64
|
1.69
|
3.54
|
3.50
|
1.46
|
0.22
|
3.13
|
2.38
|
|
[MOIM][BF4][17]
|
10.01
|
7.04
|
0.83
|
1.17
|
1.71
|
1.29
|
1.48
|
0.85
|
0.15
|
1.33
|
1.00
|
|
[MOIM] [Tf2N] [16]
|
7.89
|
5.52
|
1.75
|
-
|
1.57
|
-
|
-
|
1.54
|
0.31
|
-
|
-
|
|
[C16MIM][BF4][18]
|
3.10
|
2.13
|
1.52
|
0.76
|
1.35
|
1.29
|
1.47
|
1.27
|
0.55
|
1.08
|
0.96
|
|
[3C6C14P][BF4]*
|
3.41
|
2.38
|
1.31
|
0.92
|
1.26
|
1.25
|
1.30
|
2.44
|
0.90
|
2.32
|
2.25
|
|
[3C6C14P] [Tf2N]*
|
2.80
|
2.03
|
2.77
|
1.23
|
1.21
|
3.80
|
4.00
|
2.56
|
1.10
|
3.51
|
3.15
|
|
[3C6C14P][(C2F5)3PF3][20]
|
3.25
|
2.40
|
5.83
|
-
|
1.23
|
-
|
-
|
5.00
|
1.89
|
-
|
-
|
|
[3C1C4N] [Tf2N] [21]
|
13.94
|
0.58
|
1.07
|
-
|
1.89
|
3.38
|
-
|
0.75
|
0.10
|
2.38
|
-
|
|
[BMPy][BF4][22][23]
|
36.88
|
17.71
|
0.66
|
1.66
|
-
|
1.52
|
1.78
|
0.61
|
-
|
1.41
|
1.01
|
|
[BMPyrr] [Tf2N] [16]
|
15.47
|
-
|
-
|
1.62
|
1.92
|
-
|
3.26
|
1.16
|
0.14
|
-
|
1.89
|
|
[Et3S] [Tf2N] [24]
|
22.71
|
12.76
|
1.28
|
-
|
2.17
|
-
|
-
|
0.90
|
0.09
|
-
|
-
|
|
[Epy] [Tf2N] [25][26]
|
24.38
|
14.31
|
0.95
|
2.00
|
2.33
|
2.62
|
2.65
|
0.77
|
0.07
|
2.13
|
1.54
|
|
[3C6C14P][PF6]*
|
2.96
|
2.15
|
3.12
|
0.97
|
1.20
|
3.17
|
3.21
|
1.47
|
0.59
|
1.49
|
1.42
|
|
[C13C8N] [Tf2N] [27]*
|
3.77
|
2.70
|
2.68
|
1.22
|
1.33
|
3.11
|
3.44
|
2.27
|
0.80
|
2.63
|
2.78
|
|
[EMIM][TfO][28]*
|
30.20
|
15.19
|
0.33
|
-
|
2.31
|
-
|
-
|
0.45
|
0.03
|
-
|
-
|
|
[MOIM][PF6][29]*
|
11.27
|
7.03
|
1.88
|
-
|
1.72
|
-
|
-
|
1.04
|
0.16
|
-
|
-
|
|
[BMIM][SbF6][30]*
|
22.25
|
12.52
|
1.41
|
1.98
|
2.08
|
4.26
|
3.74
|
0.79
|
0.07
|
2.38
|
1.56
|
|
[BMIM][PF6][31]
|
-
|
-
|
-
|
-
|
3.06
|
3.06
|
-
|
-
|
-
|
1.41
|
1.01
|
|
[EMIM][TFA][32]
|
27.03
|
12.97
|
0.08
|
-
|
2.34
|
-
|
-
|
0.36
|
0.03
|
-
|
-
|
|
[HMIM][TfO][33]
|
14.37
|
7.37
|
-
|
-
|
-
|
-
|
-
|
0.68
|
-
|
-
|
-
|
|
[BMPyrr][TfO][34]
|
-
|
-
|
-
|
2.45
|
-
|
-
|
-
|
-
|
0.05
|
-
|
-
|
|
[HMPyrr] [Tf2N] [35]
|
-
|
-
|
-
|
1.70
|
-
|
-
|
-
|
-
|
0.22
|
-
|
-
|
|
[OMPyrr] [Tf2N] [35]
|
-
|
-
|
-
|
1.53
|
-
|
-
|
-
|
-
|
0.30
|
-
|
-
|
|
Sulfolane[36]
|
18.17
|
9.81
|
0.91
|
1.20
|
-
|
1.38
|
1.52
|
0.43
|
-
|
0.64
|
0.51
|
|
NMP[37]
|
11.24
|
7.05
|
-
|
-
|
0.53
|
-
|
-
|
0.95
|
0.16
|
-
|
-
|
|
Chlorobenzene[38][39]
|
-
|
-
|
-
|
-
|
-
|
0.17*
|
8.29
|
-
|
-
|
-
|
0.47
|
|
Dimethylsulfoxide[38][39]
|
-
|
-
|
-
|
-
|
-
|
0.35*
|
0.20*
|
-
|
-
|
0.38*
|
0.35*
|
Appendix G: Effect of structure on infinite dilution activity
coefficient values
| 


