APPENDIX G: EFFECT OF STRUCTURE ON IDAC VALUES
1. Infinite dilution activity coefficients of alkanes in
fluorinated ionic liquids.
1.1. Infinite dilution activity coefficients of alkanes
in imidazolium-based fluorinated ionic liquids.
1.1.1. Effect of the cation
|
1n(L413)
|
7 6 5 4
3 2 1 0
|
|
[EMIM] [BF4] [1] ? [BMIM] [BF4] [6]
[MMPIM] [BF4] [10]
[HMIM] [BF4] [12] ? [MOIM] [BF4] [17] O
[C16MIM] [BF4] [18]
|
4 5 6 7 8 9
Nc
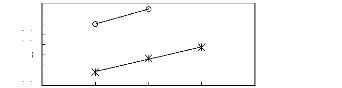
Figure G-1: Plots of versus Nc for alkanes in
imidazolium-based FILs comprising [BF4] -
ion.
|
lii(LP13)
|
4.5
4
3.5
3
2.5
2
1.5
1
|
|
[EMIM] [Tf2N] [3]
[MMIM] [Tf2N] [4] ? [BMIM] [Tf2N] [4]
O [EMMIM] [Tf2N] [3] ? [HMIM] [Tf2N] [14]
[OMIM] [Tf2N] [16]
|
|
4 5 6 7 8 9
|
|
Nc
Figure G-2: Plots of versus Nc for alkanes in
imidazolium-based FILs comprising [Tf2N]-
ion.
1n(L13)
|
3.5
3
2.5
2
|
|
[HMIM] [PF6] [13] ? [OMIM] [PF6] [29]
|
|
4 5 6 7 8 9
Nc
Figure G-3: Plots of versus Nc for alkanes in
imidazolium-based FILs comprising [PF6] -
ion.
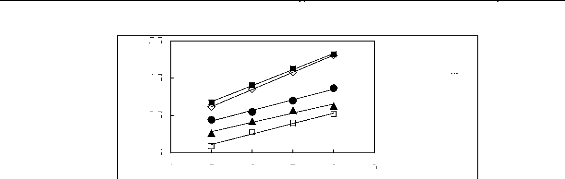
5.5
4.5
3.5
2.5
1n(L413)
? [BMIM] [TfO] [9] [EMIM] [TfO] [28] ?
[BMIM] [SbF6] [30] [EMIM] [TFA] [32]
4 5 6 7 8 9
Nc
Figure G4: Plots of versus Nc for alkanes in
imidazolium-based FILs comprising [TfO]-,
[SbF6] - and [TFA]- ions.
1.1.2. Effect of the anion
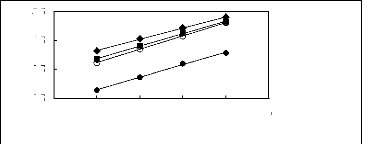
[EMIM] [BF4] [1] ? [EMIM] [Tf2N][3] O
[EMIM] [TfO] [28] [EMIM] [TFA] [32]
1n(L13)
4.5
3.5
2.5
5.5
4 5 6 7 8 9
Nc
Figure G-5: Plots of versus Nc for alkanes in
imidazolium-based FILs comprising
[EMIM] + ion.
|
1n(L13)
|
5 4 3 2
|
|
[BMIM] [BF4] [6] ? [BMIM] [Tf2N][4]
[BMIM] [TfO] [9] ? [BMIM] [SbF6] [30]
|
|
4 5 6 7 8 9
|
|
Nc
Figure G-6: Plots of versus Nc for alkanes in
imidazolium-based FILs comprising
[BMIM] + ion.
Appendix G: Effect of structure on infinite dilution activity
coefficient values
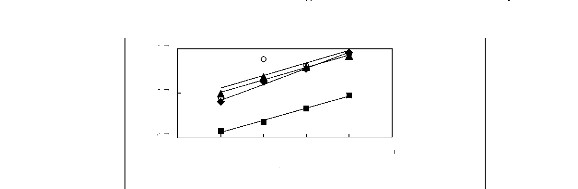
3.7
2.7
1.7
Nc
1n(L113)
4 5 6 7 8 9
O [HMIM] [BF4] [12]
· [HMIM] [PF6] [13]
· [HMIM] [Tf2N][14] ? [HMIM] [TfO]
[33]
Figure G-7: Plots of versus Nc for alkanes in
imidazolium-based FILs comprising
[HMIM] + ion.
1n(L13)
|
3.2
2.2
1.2
|
|
? [OMIM] [BF4] [17]
· [OMIM] [Tf2N][16] n [OMIM] [PF6]
[29]
|
|
|
|
|
Nc
Figure G-8: Plots of versus Nc for alkanes in
imidazolium-based FILs comprising
[MOIM] + ion.
[20]
1.2. Infinite dilution activity coefficients of alkanes
in phosphonium-based FILs
· [3C6C14P] [BF4] [19] ? [3C6C14P] [Tf2N]
[19]
· [3C6C14P] [(C2F5)3PF3]
· [3C6C14P] [PF6] [19]
|
1n(L413)
|
1.2
0.8
0.4
0 -0.4 -0.8
|
|
4 5 6 Nc 7 8 9
Figure G-9: Plots of versus Nc for alkanes in
phosphonium-based FILs comprising
[3C6C14P] + ion.
Appendix G: Effect of structure on infinite dilution activity
coefficient values
1.3. Infinite dilution activity coefficients of alkanes
in ammonium-based FILs
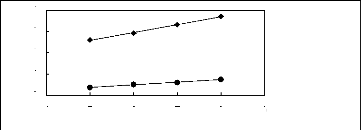
4
3
2
1n(L413)
[3C1C4N] [Tf2N] [21] ? [3C8C1N] [Tf2N]
[27]
1
0
4 5 6 7 8 9
Nc
Figure G-10: Plots of versus Nc for alkanes in
ammonium-based FILs comprising
[Tf2N] - ion.
1.4. Infinite dilution activity coefficients of alkanes
in pyridinium-based FILs
[BMPy] [BF4] [22] ? [EPy] [BTI] [25]
lii(LP13)
4.5
2.5
5.5
3.5
4 5 6 7 8 9
Nc
Figure G-11: Plots of versus Nc for alkanes in
pyridinium-based FILs.
1.5. Infinite dilution activity coefficients of alkanes
in pyrrolidinium-based FILs 1.5.1. Effect of the cation
1n(L413)
|
3.2 2.7 2.2 1.7 1.2
|
|
[BMPyrr] [Tf2N] [16]
[HMPyrr] [Tf2N] [35] O [MOPyrr] [Tf2N]
[35]
|
|
|
|
|
Nc
Figure G-12: Plots of versus Nc for alkanes in
pyrrolidinium-based FILs comprising
[Tf2N] - ion.
1.5.2. Effect of the anion
1n(L13)
|
4.2 3.2 2.2 1.2
|
|
[BMPyrr] [Tf2N] [16] ? [BMPyrr] [TfO]
[34]
|
|
4 5 6 7 8 9
Nc
Figure G-13: Plots of versus Nc for alkanes in
pyrrolidinium-based FILs comprising
[BMPyrr] + ion.
1.6. Infinite dilution activity coefficients of alkanes
in sulfonium-based FILs
4 5 6 7 8 9
Nc
? [Et3S] [Tf2N] [24]
1n(L13)
3.6
3.2
2.8
4
Figure G-14: Plot of versus Nc for alkanes in
the sulfonium-based FIL [Et3S] [Tf2N].
2. Infinite dilution activity coefficients of alk-1-enes
in fluorinated ionic liquids. 2.1. Infinite dilution activity coefficients of
alk-1-enes in imidazolium-based FILs 2.1.1. Effect of the cation
1n(L13)
|
4
3.5
3
2.5
2
1.5
|
|
[EMIM] [BF4] [2] ? [BMIM] [BF4] [2] ?
[HMIM] [BF4] [12] [MOIM] [BF4] [17]
|
|
|
|
|
Nc
Figure G-15: Plots of versus Nc for alk-1-enes
in imidazolium-based FILs comprising
[BF4]- ion.
Appendix G: Effect of structure on infinite dilution
activity coefficient values
1n(L413)
|
4.5 3.5 2.5 1.5 0.5
|
|
O [EMIM] [Tf2N] [4] ? [MMIM] [Tf2N] [4]
[BMIM] [Tf2N] [4] + [EMMIM] [Tf2N]
[3] [HMIM] [Tf2N] [14] ? [MOIM] [Tf2N]
[16]
|
|
|
|
|
Nc
Figure C-16: Plots of versus Nc for alk-1-enes
in imidazolium-based FILs comprising
[Tf2N]- ion.
4 5 6 7 8 9
Nc
[HMIM] [PF6] [13] ? [MOIM] [PF6] [29]
lii(LP13)
4
2
3
0
1
Figure C-17: Plots of versus Nc for alk-1-enes
in imidazolium-based FILs comprising
[PF6]- ion.
4 5 6 7 8 9
Nc
[EMIM] [TfO] [28] ? [BMIM] [TfO] [9]
1n(L13)
4
2
6
8
Figure C-18: Plots of versus Nc for alk-1-enes
in imidazolium-based FILs comprising
[TfO]- ion.
2.1.2. Effect of the anion
1n(L13)
|
4.5
3
1.5
|
|
· [EMIM] [BF4] [2] n [EMIM] [Tf2N]
[4] O [EMIM] [TfO] [28] A [EMIM] [TFA] [32]
|
|
|
|
|
Nc
Figure G-19: Plots of versus Nc for alk-1-enes
in imidazolium-based FILs comprising
[EMIM] + ion.
|
5
4 3 2 1 0
|
|
|
1n(L113)
|
|
· [BMIM] [BF4] [2] A [BMIM] [Tf2N]
[4]
· [BMIM] [TfO] [9] O [BMIM] [SbF6]
[30]
|
|
|
|
|
4 5 6 7 8 9
Nc
Figure G-20: Plots of versus Nc for alk-1-enes
in imidazolium-based FILs comprising
[BMIM] + ion.
ln(L413)
|
2.8 2.3 1.8 1.3 0.8
|
|
A [HMIM] [BF4] [12]
O [HMIM] [PF6]
[13]
· [HMIM] [Tf2N] [14]
|
|
4 5 6 7 8 9
Nc
Figure G-21: Plots of versus Nc for alk-1-enes
in imidazolium-based FILs comprising
[HMIM] + ion.
Appendix G: Effect of structure on infinite dilution
activity coefficient values
1n(L413)
|
2.8 2.3 1.8 1.3 0.8
|
|
· [MOIM] [BF4] [17] ? [MOIM] [Tf2N]
[16]
· [MOIM] [PF6] [29]
|
|
|
|
|
Nc
Figure G-22: Plots of versus Nc for alk-1-enes
in imidazolium-based FILs comprising
[MOIM] + ion.
2.2. Infinite dilution activity coefficients of
alk-1-enes in phosphonium-based FILs
Ini3)
|
1.2 0.7 0.2 -0.3 -0.8
|
|
· [3C6C14P] [BF4] [19]
· [3C6C14P] [Tf2N] [19]
· [3C6C14P] [(C2F5)3PF3] [20] ? [3C6C14P] [PF6]
[19]
|
|
|
|
|
Nc
Figure G-23: Plots of versus Nc for alk-1-enes
in phosphonium-based FILs comprising
[3C6C14P] + ion.
2.3. Infinite dilution activity coefficients of
alk-1-enes in ammonium-based FILs
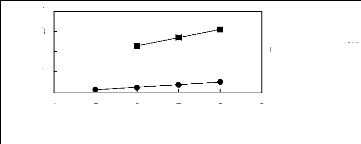
1n(L13)
4
2
3
0
1
4 5 6 7 8 9
Nc
· [3C1C4N] [Tf2N] [21]
· [3C8C1N] [Tf2N] [27]
Figure G-24: Plots of versus Nc for alk-1-enes
in ammonium-based FILs comprising
[Tf2N] ion.
2.4. Infinite dilution activity coefficients of
alk-1-enes in pyridinium-based FILs
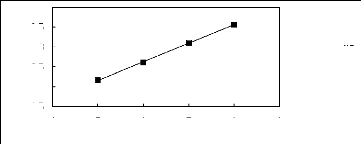
4 5 6 7 8 9
Nc
. [EPy] [Tf2N] [25]
1n(L113)
2.5
3.5
1.5
4
2
3
Figure G-25: Plot of versus Nc for alk-1-enes in
the pyridinium-based Fl L [Epy] [Tf2N].
2.5. Infinite dilution activity coefficients of
alk-1-enes in pyrrolidinium-based FILs 2.5.1. Effect of the cation
1n(L113)
|
3
2.5
2
1.5
1
0.5
0
|
|
· [BMPyrr] [Tf2N] [16] ? [HMPyrr] [Tf2N]
[35]
· [MOPyrr] [Tf2N] [35]
|
|
4 5 6 7 8 9
Nc
Figure G-26: Plots of versus Nc for alk-1-enes
in pyrrolidinium-based Fl Ls comprising
[Tf2N] - ion.
2.5.2. Effect of the anion
|
1n(L113)
|
4
3
2
1
|
|
· [BMPyrr] [Tf2N] [16]
· [BMPyrr] [TfO] [34]
|
4 5 6 7 8 9
Nc
Figure G-27: Plots of versus Nc for alk-1-enes
in pyrrolidinium-based Fl Ls comprising
[BMPyrr] + ion.
1.2.7. Infinite dilution activity coefficients of
alk-1-enes in sulfonium-based FILs
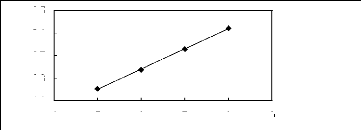
4 5 6 7 8 9
Nc
[Et3S] [Tf2N] [24]
Ini3)
3.5
3.1
2.7
2.3
1.9
Figure G-28: Plot of versus Nc for alk-1-enes in
the sulfonium-based FIL [Et3S] [Tf2N].
3. Infinite dilution activity coefficients of alk-1-ynes
in fluorinated ionic liquids. 3.1. Infinite dilution activity coefficients of
alk-1-ynes in imidazolium-based FILs 3.1.1. Effect of the cation
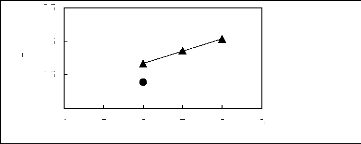
4 5 6 7 8 9
Nc
? [HMIM] [BF4] [12] ? [C16MIM] [BF4]
[18]
ln(L13)
-0.5
2.5
0.5
1.5
Figure G-29: Plots of versus Nc for alk-1-ynes
in imidazolium-based FILs comprising
[BF4] - ion.
|
2
1.5
1
0.5
0
|
|
|
|
in(Li3)
|
|
[HMIM] [PF6] [13]
? [MOIM] [PF6]
[29]
|
|
|
|
4 5 6 7 8 9
Nc
Figure G-30: Plots of versus Nc for alk-1-ynes
in imidazolium-based FILs comprising
[PF6] - ion.
|
1.8
1.4
1 0.6 0.2
|
|
|
|
1n(L113)
|
|
[EMIM] [Tf2N] [5] ? [HMIM] [Tf2N] [15]
|
|
|
|
4 5 6 7 8 9
Nc
Figure G-31: Plots of versus Nc for alk-1-ynes
in imidazolium-based FILs comprising
[Tf2N] - ion.
|
1n(L413)
|
2.5
1.5
0.5
|
|
? [BMIM] [TfO] [9] O [EMIM] [TfO] [28]
|
|
|
|
4 5 6 7 8 9
Nc
Figure G-32: Plots of versus Nc for alk-1-ynes
in imidazolium-based FILs comprising
[TfO] - ion.
3.1.2. Effect of the anion
|
1n(L413)
|
3 2 1 0
|
|
[EMIM] [Tf2N] [5] ? [EMIM] [TfO] [28] O
[EMIM] [TFA] [32]
|
4 5 6 7 8 9
Nc
Figure G-33: Plots of versus Nc for alk-1-ynes
in imidazolium-based FILs comprising
[EMIM] + ion.
Appendix G: Effect of structure on infinite dilution activity
coefficient values
|
2.5
2
1.5
1
0.5
|
|
|
|
1n(L13)
|
|
? [BMIM] [TfO] [9] ? [BMIM] [SbF6] [30]
|
|
|
|
4 5 6 7 8 9
Nc
Figure G-34: Plots of versus Nc for alk-1-ynes
in imidazolium-based FILs comprising
[BMIM] + ion.
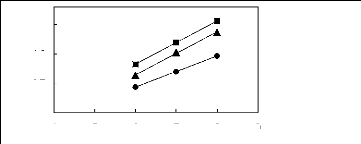
4 5 6 7 8 9
Nc
? [HMIM] [BF4] [12] [HMIM] [PF6] [13] ?
[HMIM] [Tf2N] [15]
in(L13)
0.7
0.2
1.7
1.2
Figure G-35: Plots of versus Nc for alk-1-ynes
in imidazolium-based FILs comprising
[HMIM] + ion.
3.2. Infinite dilution activity coefficients of
alk-1-ynes in phosphonium-based FILs
|
1n( L13)
|
0.4
0
-0.4
-0.8
-1.2
|
|
[3C6C14P] [BF4] [19] ? [3C6C14P] [Tf2N]
[19]
? [3C6C14P] [(C2F5)3PF3] [20] [3C6C14P] [PF6]
[19]
|
4 5 6 7 8 9
Nc
Figure G-36: Plots of versus Nc for alk-1-ynes
in phosphonium-based FILs comprising
[3C6C14P] + ion.
Appendix G: Effect of structure on infinite dilution activity
coefficient values
3.3. Infinite dilution activity coefficients of
alk-1-ynes in ammonium, pyrrolidinium and sulfonium-based FILs
1n(L113)
|
3 2 1 0 -1
|
|
[Et3S] [Tf2N] [24]
? [C13C8N] [Tf2N] [27] ? [BMPyrr] [TfO]
[34]
|
|
4 5 6 7 8 9
Nc
Figure G-37: Plots of versus Nc for alk-1-ynes
in an ammonium, a pyrrolidinium and a
sulfonium-based FILs.
4. Infinite dilution activity coefficients of
cycloalkanes in fluorinated ionic liquids. 4.1. Infinite dilution activity
coefficients of cycloalkanes in imidazolium-based FILs
4.1.1. Effect of the cation
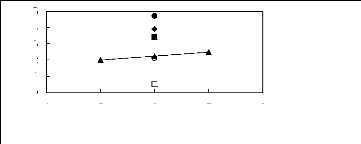
[EMIM] [BF4] [2] [BMIM] [BF4] [6] ?
[MMPIM] [BF4] [10] ? [HMIM] [BF4] [12] O [MOIM] [BF4]
[17] ? [C16MIM] [BF4] [18]
4 5 6 7 8
Nc
1n(L13)
4
2
5
3
0
1
Figure G-38: Plots of versus Nc for cycloalkanes
in imidazolium-based FILs comprising
[BF4]- ion.
|
4.5 3.5 2.5 1.5 0.5
|
|
|
|
1n(L413)
|
|
[EMIM] [Tf2N] [5] ? [MMIM] [Tf2N] [4] ?
[BMIM] [Tf2N] [4]
[EMMIM] [Tf2N] [3] ? [HMIM] [Tf2N]
[15] O [MOIM] [Tf2N] [16]
|
|
|
|
4 5 6 7 8 9
Nc
Figure G-39: Plots of versus Nc for cycloalkanes
in imidazolium-based FILs comprising
[Tf2N] - ion.
Appendix G: Effect of structure on infinite dilution activity
coefficient values
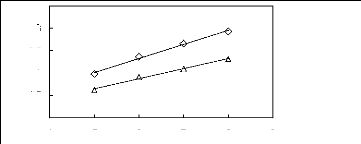
4 5 6 7 8 9
Nc
O [HMIM] [PF6] [13]
A [MOIM] [PF6]
[29]
ln(LI13)
2.5
3.5
1.5
2
3
1
Figure G-40: Plots of versus Nc for cycloalkanes
in imidazolium-based FILs comprising
[PF6] - ion.
1n(L13)
|
4.5
4
3.5
3
2.5
2
|
|
· [BMIM] [TfO] [9] O [BMIM] [TfO]
[28]
· [HMIM] [TfO] [33]
|
|
4 5 6 7 8 9
Nc
Figure G-41: Plots of versus Nc for cycloalkanes
in imidazolium-based FILs comprising
[TfO] - ion.
4.1.2. Effect of the anion
|
ln(LI13)
|
4.5
4
3.5
3
2.5
2
|
|
· [EMIM] [BF4] [2] n [EMIM] [Tf2N]
[5] O [EMMIM] [Tf2N]] [3]
· [EMIM] [TfO] [28] A [EMIM] [TFA]
[32]
|
4 5 6 7 8 9
Nc
Figure G-42: Plots of versus Nc for cycloalkanes
in imidazolium-based FILs comprising
[EMIM] + ion.
Appendix G: Effect of structure on infinite dilution activity
coefficient values
ã 1 3
|
3.7 3.2 2.7 2.2 1.7 1.2
|
|
|
1n(L13)
|
|
[BMIM] [BF4] [6] [BMIM] [Tf2N] [4] O
[BMIM] [TfO] [9] ? [BMIM] [SbF6] [30]
|
|
|
|
|
4 5 6 7 8 9
Nc
Figure G-43: Plots of versus Nc for cycloalkanes
in imidazolium-based FILs comprising
[BMIM] + ion.
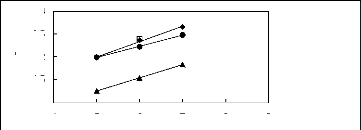
3
2.5
2
1.5
1
in(L13)
? [HMIM] [BF4] [12]
[HMIM] [PF6] [13] ?
[HMIM] [Tf2N] [15] ? [HMIM] [TfO] [33]
4 5 6 7 8 9
Nc
Figure G-44: Plots of versus Nc for cycloalkanes
in imidazolium-based FILs comprising
[HMIM] + ion.
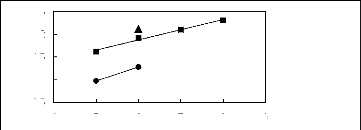
4 5 6 7 8 9
Nc
? [MOIM] [BF4] [17] ? [MOIM] [Tf2N]
[16]
in(L413)
2.5
0.5
1.5
2
1
Figure G-45: Plots of versus Nc for cycloalkanes
in imidazolium-based FILs comprising
[MOIM] + ion.
Appendix G: Effect of structure on infinite dilution
activity coefficient values
4.2. Infinite dilution activity coefficients of
cycloalkanes in phosphonium-based FILs
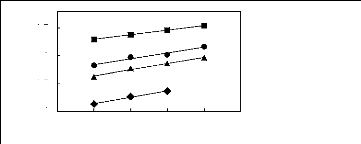
0.5
0
-0.5
-1
1n(L413)
? [3C6C14P] [BF4] [19]
? [3C6C14P] [Tf2N] [19]
[3C6C14P] [(C2F5)3PF3] [20] [3C6C14P] [PF6]
[19]
4 5 6 7 8 9
Nc
Figure G-46: Plots of versus Nc for cycloalkanes
in phosphonium-based FILs
comprising [3C6C14P] + ion.
4.3. Infinite dilution activity coefficients of
cycloalkanes in ammonium-based FILs
|
0.6 0.4 0.2 E-15 -0.2 -0.4 -0.6
|
|
|
-1
ln(i3)
|
|
[3C1C4N] [Tf2N] [21] ? [C13C8N] [Tf2N]
[27]
|
|
|
|
|
4 5 6 7 8 9
Nc
Figure G-47: Plots of versus Nc for cycloalkanes
in ammonium-based FILs comprising
[Tf2N] - ion.
4.4. Infinite dilution activity coefficients of
cycloalkanes in pyridinium-based FILs
|
4
3.5
3
2.5
2
|
|
|
1n(L13)
|
|
[BMPy] [BF4] [22] ? [EPy] [Tf2N] [25]
|
|
|
|
|
4 5 6 7
Nc
Figure G-48: Plots of versus Nc for cycloalkanes
in the pyridinium-based FILs
[Epy] [Tf2N] and [BMPy] [BF4].
Appendix G: Effect of structure on infinite dilution
activity coefficient values
4.5. Infinite dilution activity coefficients of
cycloalkanes in pyrrolidinium-based FILs
|
4
3.5
3
2.5 2 1.5
|
|
|
1n(L13)
|
|
[BMPyrr] [Tf2N] [6] ? [BMPyrr] [TfO]
[34]
|
|
|
|
|
4 5 6 7 8 9
Nc
Figure C-49: Plots of versus Nc for cycloalkanes
in ammonium-based FILs comprising
[BMPyrr] + ion.
4.6. Infinite dilution activity coefficients of
cycloalkanes in sulfonium-based FILs
in(1113)
|
3.2
2.8
2.4
2
|
|
? [Et3S] [Tf2N] [24]
|
|
|
|
|
4 5 6 7 8 9
Nc
Figure C-50: Plots of versus Nc for cycloalkanes
in the sulfonium-based FIL [Et3S]
[Tf2N].
5. Infinite dilution activity coefficients of
alkan-1-ols in fluorinated ionic liquids. 5.1. Infinite dilution activity
coefficients of alkan-1-ols in imidazolium-based FILs 5.1.1. Effect of the
cation
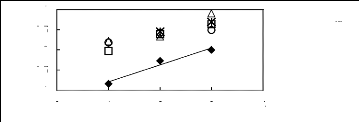
0 1 2 3 4
Nc
[EMIM] [BF4] [2] ? [BMIM] [BF4] [7] *
[HMIM] [BF4] [2] ? [MOIM] [BF4] [17] O [C16MIM] [BF4]
[18]
In(L13)
-0.5
0.5
-1
0
1
Figure C-51: Plots of versus Nc for alkan-1-ols
in imidazolium-based FILs comprising
[BF4]-ion.
Appendix G: Effect of structure on infinite dilution activity
coefficient values
1n(L413)
|
1.2
1 0.8 0.6 0.4 0.2
0
|
|
· [EMIM] [Tf2N] [4] O [BMIM] [Tf2N]
[8]
· [EMMIM] [Tf2N] [3] O [HMIM] [Tf2N]
[16] A [MOIM] [Tf2N] [16]
|
|
|
|
|
Nc
Figure C-52: Plots of versus Nc for alkan-1-ols
in imidazolium-based FILs comprising
[Tf2N]- ion.
1n(L413)
|
1.6
1.4
1.2
1 0.8 0.6 0.4 0.2
0
|
|
· [HMIM] [PF6] [13]
· [MOIM] [PF6] [29]
· [BMIM] [PF6] [31]
|
|
0 1 2 3 4
Nc
Figure C-53: Plots of versus Nc for alkan-1-ols
in imidazolium-based FILs comprising
[PF6]- ion.
0 1 2 3 4
Nc
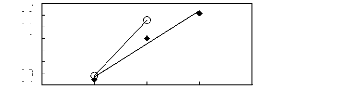
· [BMIM] [TfO] [9] O [EMIM] [TfO]
[28]
1n(L413)
-0.1
-0.2
-0.3
-0.4
0.3
0.2
0.1
0
Figure C-54: Plots of versus Nc for alkan-1-ols
in imidazolium-based FILs comprising
[TfO]- ion.
5.1.2. Effect of the anion
in(L413)
|
1
0.5
0
-0.5
-1
-1.5
-2
|
|
· [EMIM] [BF4] [4] n [EMIM] [Tf2N]
[28]
· [EMIM] [TfO] [32] * [EMIM] [TFA]
[2]
|
|
|
|
|
Nc
Figure C-55: Plots of versus Nc for alkan-1-ols
in imidazolium-based FILs comprising
[EMIM] + ion.
1n(L13)
|
1.5
1
0.5
0
-0.5
|
|
A [BMIM] [BF4] [7] O [BMIM] [Tf2N] [8]
· [BMIM] [TfO] [9]
· [BMIM] [SbF6] [30]
· [BMIM] [PF6] [31]
|
|
|
|
|
Nc
Figure C-56: Plots of versus Nc for alkan-1-ols
in imidazolium-based FILs comprising
[BMIM] + ion
0 1 2 3 4
Nc
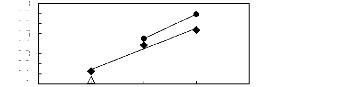
· [HMIM] [BF4] [2] A [HMIM] [PF6]
[13]
· [HMIM] [Tf2N] [16]
in(L413)
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0
Figure C-57: Plots of versus Nc for alkan-1-ols
in imidazolium-based FILs comprising
[HMIM] + ion.
Appendix G: Effect of structure on infinite dilution activity
coefficient values
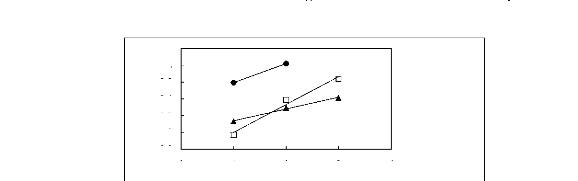
0 1 2 3 4
Nc
n [MOIM] [BF4] [17] ? [MOIM] [Tf2N] [16] .
[MOIM] [PF6] [21]
1
0.8
0.6
1n(L13)
0.4
0.2
0
-0.2
Figure G-58: Plots of versus Nc for alkan-1-ols
in imidazolium-based FILs comprising
[EMIM] + ion.
5.2. Infinite dilution activity coefficients of
alkan-1-ols in phosphonium-based FILs
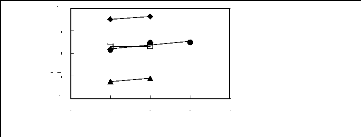
1
0.5
0
-0.5
-1
lii(LP13)
? [3C6C14P] [BF4] [19]
· [3C6C14P] [Tf2N] [19]
n [3C6C14P] [(C2F5)3PF3] [20]
· [3C6C14P] [PF6] [19]
Nc
0 1 2 3 4
Figure G-59: Plots of versus Nc for alkan-1-ols
in phosphonium-based FILs comprising
[3C6C14P] + ion.
5.3. Infinite dilution activity coefficients of
alkan-1-ols in ammonium-based FILs
0 1 2 3 4
Nc
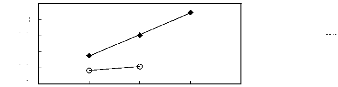
· [3C1C4N] [Tf2N] [21] O [C13C8N] [Tf2N]
[27]
1n(L13)
0.8
0.6
0.4
0.2
0
1
Figure G-60: Plots of versus Nc for alkan-1-ols
in ammonium-based FILs comprising
[Tf2N]- ion.
Appendix G: Effect of structure on infinite dilution activity
coefficient values
5.4. Infinite dilution activity coefficients of
alkan-1-ols in pyridinium-based FILs
0 1 2 3 4
Nc
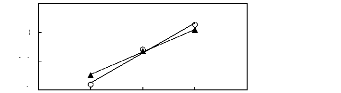
O [BMPy] [BF4] [23] ? [EPy] [Tf2N] [25] [26]
1n(L413)
0.8
0.4
1.2
0
Figure G-61: Plots of versus Nc for alkan-1-ols
in the pyridinium-based FILs
[BMPy] [BF4] and [Epy] [Tf2N].
5.5. Infinite dilution activity coefficients of
alkan-1-ols in pyrrolidinium-based FILs
|
0.6
0.4
|
|
|
|
|
|
|
1n(L113)
|
0.2
0
-0.2
-0.4
-0.6
|
|
[BMPyrr] [Tf2N] [16] ? [BMPyrr] [TfO]
[34]
|
|
|
|
0 1 2 3 4
Nc
Figure G-62: Plots of versus Nc for alkan-1-ols
in pyrrolidinium-based FILs comprising
[BMPyrr] + ion.
5.6. Infinite dilution activity coefficients of
alkan-1-ols in sulfonium-based FILs
|
1
0.5
0
|
|
|
|
1n(L13)
|
|
[Et3S] [Tf2N] [24]
|
|
|
|
0 1 2 3 4
Nc
Figure G-63: Plots of versus Nc for alkan-1-ols
in the sulfonium-based FILs
[Et3S] [Tf2N].
6. Infinite dilution activity coefficients of
alkylbenzenes in fluorinated ionic liquids
6.1. Infinite dilution activity coefficients of
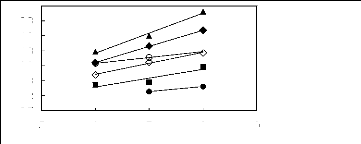
alkylbenzenes in imidazolium-based FILs 6.1.1. Effect of the cation
2.8
2.3
1.8
1.3
0.8
0.3
-0.2
-0.7
lii(LP13)
· [EMIM] [BF4] [2] O [BMIM] [BF4] [2]
A [MMPIM] [BF4] [10] O [HMIM] [BF4] [2]
· [MOIM] [BF4] [17]
· [C16MIM] [BF4] [18]
5 6 7 8 9
Nc
Figure G-64: Plots of versus Nc for
alkylbenzenes in imidazolium-based FILs
comprising [BF4]- ion.
ln(L13)
|
1.3 0.8 0.3 -0.2 -0.7
|
|
· [EMIM] [Tf2N] [4] A [MMIM] [Tf2N]
[4] A [BMIM] [Tf2N] [8] O [EMMIM] [Tf2N]
[3]
· [HMIM] [Tf2N] [16]
· [MOIM] [Tf2N] [16]
|
|
|
|
|
Nc
Figure G-65: Plots of versus Nc for
alkylbenzenes in imidazolium-based FILs
comprising [Tf2N]- ion.
5 6 7 8 9
Nc
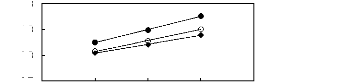
O [BMIM] [TfO] [9]
· [EMIM] [TfO] [28]
· [HMIM] [TfO] [33]
ln(L413)
-0.7
2.3
0.3
1.3
Figure G-66: Plots of versus Nc for
alkylbenzenes in imidazolium-based FILs
comprising [TfO]- ion.
6.1.2. Effect of the anion
5 6 7 8 9
Nc
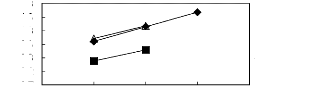
· [EMIM] [BF4] [2]
· [EMIM] [Tf2N] [4] A [EMIM] [TFA]
[32]
1n(L13)
-0.2
-0.7
2.3
0.8
0.3
1.8
1.3
Figure C-67: Plots of versus Nc for
alkylbenzenes in imidazolium-based FILs
comprising [EMIM] + ion.
|
1.8 1.3 0.8 0.3 -0.2 -0.7
|
|
|
|
ln(L13)
|
|
n [BMIM] [BF4] [2] * [BMIM] [Tf2N] [8] O
[BMIM] [TfO] [9] A [BMIM] [SbF6] [30]
|
|
|
|
5 6 7 8 9
Nc
Figure C-68: Plots of versus Nc for
alkylbenzenes in imidazolium-based FILs
comprising [BMIM] + ion.
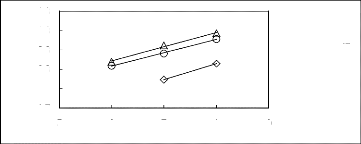
5 6 7 8 9
Nc
A [HMIM] [BF4] [2] O [HMIM] [Tf2N] [16]
O[HMIM] [TfO] [33]
ln(L113)
-0.2
-0.7
0.8
0.3
1.8
1.3
Figure C-69: Plots of versus Nc for
alkylbenzenes in imidazolium-based FILs
comprising [HMIM] + ion.
5 6 7 8 9
Nc
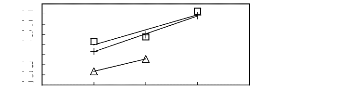
? [MOIM] [BF4] [17] ? [MOIM] [Tf2N] [16]
+ [MOIM] [PF6] [29]
0.9
0.7
0.5
1n(L413)
0.3
0.1
-0.1
-0.3
-0.5
-0.7
Figure G-70: Plots of versus Nc for
alkylbenzenes in imidazolium-based FILs
comprising [MOIM] + ion.
6.2. Infinite dilution activity coefficients of
alkylbenzenes in phosphonium-based FILs
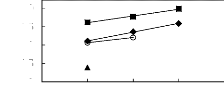
[3C6C14P] [BF4] [19] O [3C6C14P] [Tf2N]
[19] ? [3C6C14P] [(C2F5)3PF3] [20] [3C6C14P] [PF6]
[19]
5 6 7 8 9
Nc
1n(L13)
-0.5
-1.5
-1
-2
0
Figure G-71: Plots of versus Nc for
alkylbenzenes in phosphonium-based FILs
comprising [3C6C14P]+ ion.
6.3. Infinite dilution activity coefficients of
alkylbenzenes in ammonium-based FILs
5 6 7 8 9
Nc
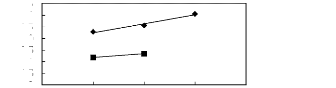
[3C1C4N] [BTI] [21] [C13C8N] [BTI]
[27]
1n(L13)
-0.5
-1.5
0.5
1.5
-1
-2
0
1
Figure G-72: Plots of versus Nc for
alkylbenzenes in ammonium-based FILs comprising
[Tf2N]- ion.
6.4. Infinite dilution activity coefficients of
alkylbenzenes in pyridinium-based FILs
|
1.1 0.9 0.7 0.5 0.3 0.1 -0.1 -0.3 -0.5
|
|
|
|
In(L13)
|
|
? [BMPy] [BF4] [22] ? [EPy] [Tf2N] [25]
|
|
|
|
5 6 7 8
Nc
Figure G-73: Plots of versus Nc for
alkylbenzenes in the pyridinium-based FILs
[BMPy] [BF4] and [Epy] [Tf2N].
6.5. Infinite dilution activity coefficients of
alkylbenzenes in pyrrolidinium and sulfoniumbased FILs
|
2
1
0
-1
-2
|
|
|
|
lii(L113)
|
|
[BMPyrr] [Tf2N] [16] [Et3S] [Tf2N]
[24]
|
|
|
|
5 6 7 8 9
Nc
Figure G-74: Plots of versus Nc for
alkylbenzenes in [BMPyrr] [Tf2N] and [Et3S] [Tf2N].
7. Infinite dilution activity coefficients of ket-2-ones
in fluorinated ionic liquids 7.1. Infinite dilution activity coefficients of
ket-2-ones in imidazolium-based FILs 7.1.1. Effect of the cation
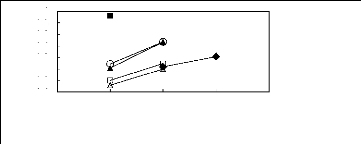
O [EMIM] [BF4] [2]
A [BMIM] [BF4]
[2]
· [MMPIM] [BF4] [11] n [HMIM] [BF4]
[2] A [MOIM] [BF4] [17]
· [C16MIM] [BF4] [18]
2 3 4 5 6
Nc
1
0.8
0.6
0.4
0.2
0
-0.2
-0.4
1n(L13)
Figure C-75: Plots of versus Nc for ket-2-ones
in imidazolium-based FILs comprising
[BF4] - ion.
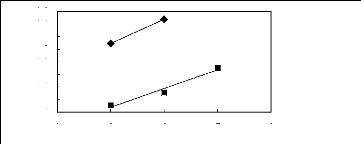
1n( L13)
-0.2
-0.4
-0.6
-0.8
-1.2
-1
0
2 3 4 5 6
Nc
n [EMIM] [[Tf2N]] [4] O [EMMIM] [Tf2N]
[3] A [HMIM] [Tf2N] [16]
Figure C-76: Plots of versus Nc for ket-2-ones
in imidazolium-based FILs comprising
[Tf2N] - ion.
7.1.2. Effect of the anion
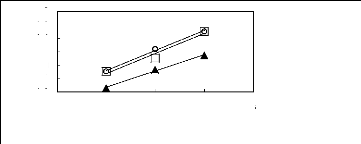
2 3 4 5 6
Nc
· [EMIM] [BF4] [2]
· [EMIM] [Tf2N] [4]
1n( L13)
-0.2
-0.4
-0.6
-0.8
0.6
0.4
0.2
-1
0
Figure C-77: Plots of versus Nc for ket-2-ones
in imidazolium-based FILs comprising
[EMIM] + ion.
Appendix G: Effect of structure on infinite dilution activity
coefficient values
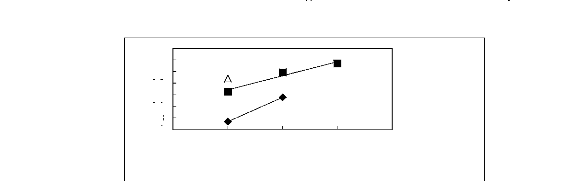
0.4
0.2
0
ln(LI13)
-0.2
-0.4
-0.6
-0.8
-1
A [BMIM] [TfO] [9] O [BMIM] [SbF6] [30]
· [BMIM] [PF6] [31]
2 3 4 5 6
Nc
Figure G-78: Plots of versus Nc for ket-2-ones
in imidazolium-based FILs comprising
[BMIM] + ion.
2 3 4 5 6
Nc
O [HMIM] [BF4] [17] * [HMIM] [Tf2N] [16]
1n(L13)
-0.2
-0.4
-0.6
-0.8
-1.2
-1.4
0.2
-1
0
Figure G-79: Plots of versus Nc for ket-2-ones
in imidazolium-based FILs comprising
[HMIM] + ion.
7.2. Infinite dilution activity coefficients of
ket-2-ones in phosphonium-based FILs
1n(L413)
|
0 -0.2 -0.4 -0.6 -0.8
-1 -1.2 -1.4
|
|
· [3C6C14P] [BF4] [19] A [3C6C14P]
[Tf2N] [19]
· [3C6C14P] [PF6] [19]
|
|
2 3 4 5
Nc
Figure G-80: Plots of versus Nc for ket-2-ones
in phosphonium-based FILs comprising
[3C6C14P] + ion.
7.3. Infinite dilution activity coefficients of
ket-2-ones in ammonium-based FILs
|
-0.8
-0.85
-0.9
-0.95
-1
-1.05
|
|
|
1n(L413)
|
|
· [3C1C4N] [Tf2N] [21]
· [C13C8N] [Tf2N] [27]
|
|
|
|
|
2 3 4 5
Nc
Figure G-81: Plots of versus Nc for ket-2-ones
in ammonium-based FILs comprising
[Tf2N] - ion.
7.4. Infinite dilution activity coefficients of
ket-2-ones in pyridinium-based FILs
|
0.3 0.1 -0.1 -0.3 -0.5 -0.7 -0.9
|
|
|
1n(L113)
|
|
? [BMPy] [BF4] [22] n [EPy] [Tf2N] [25]
|
|
|
|
|
2 3 4 5 6
Nc
Figure G-82: Plots of versus Nc for ket-2-ones
in the imidazolium-based FILs
[Epy] [Tf2N] and [BMPy] [BF4].
7.5. Infinite dilution activity coefficients of
ket-2-ones in pyrrolidinium-based FILs
|
-0.2 -0.3 -0.4 -0.5 -0.6 -0.7
|
|
|
|
1n(L413)
|
|
· [BMPyrr] [Tf2N] [16]
|
|
|
|
3 4 5 6
Nc
Figure G-83: Plot of versus Nc for ket-2-ones in
the pyrrolidinium-based FIL [BMPyrr]
[Tf2N].
APPENDIX H: EFFECT OF STRUCTURE ON
LIMITING
SELECTIVITY AND CAPACITY
1. Benzene/n-hexane separation problem
1.1. Imidazolium-based fluorinated ionic
liquids
Limiting Selectivity
|
50 40 30 20 10 0
|
|
[EMIM] + [BMIM] + ? [HMIM] + ? [MOIM] + O [C16MIM] +
|
|
|
|
|
Anions
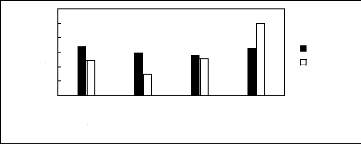
Figure H-1: Limiting selectivity at 313.15 K
of imidazolium-based fluorinated ionic liquids for
the hexane (1)/benzene
(2) system, representing aliphatics/aromatics separation
problems.
[EMIM][BF4][1][2];[EMIM] [Tf2N] [3][4];
[BMIM][BF4][2][6] ; [BMIM] [Tf2N] [4][8] ;
[BMIM][TfO] [9] ;
[HMIM][BF4] [12][2] ; [HMIM][PF6] [13] ; [HMIM] [Tf2N] [14][16]
;
[MOIM][BF4] [17] ; [MOIM] [Tf2N] [16] ; [C16MIM][BF4] [18] ; [EMIM][TfO]
[28]; [MOIM][PF6]
[29]; [BMIM][SbF6] [30] ; [BMIM][PF6] [31] ;
[EMIM][TFA] [32] ; [HMIM][TfO] [33].
|
2
1.5
1
0.5
0
|
|
|
|
Limiting Capacity
|
|
[EMIM] + ? [BMIM] + [HMIM] + ? [MOIM] + O [C16MIM] +
|
|
[BF4]- [PF6] - [TfO] - [SbF6] - [TFA] - [Tf2N]-
|
|
Anions
Figure H-2: Limiting capacity at 313.15 K of
imidazolium-based fluorinated ionic liquids for
the hexane (1)/benzene (2)
system, representing aliphatics/aromatics separation
problems.
[EMIM][BF4][2];[EMIM] [Tf2N] [4];
[BMIM][BF4][6] ; [BMIM] [Tf2N] [8] ; [BMIM][TfO] [9]
;
[HMIM][BF4] [2] ; [HMIM][PF6] [13] ; [HMIM] [Tf2N] [16] ; [MOIM][BF4] [17]
; [MOIM]
[Tf2N] [16] ; [C16MIM][BF4] [18] ; [EMIM][TfO] [28];
[MOIM][PF6] [29]; [BMIM][SbF6] [30] ;
[BMIM][PF6] [31] ;
[EMIM][TFA] [32] ; [HMIM][TfO] [33].
1.2. Phosphonium-based fluorinated ionic
liquids
Limiting Selectivity
and Capacity
4
2
6
3
0
5
1
[3C6C14P][BF4] [3C6C14P][PF6] [3C6C14P]
[Tf2N] [3C6C14P][(C2F5)2PF3]
Ionic liquids with a common cation
Selectivity Capacity
Figure H-3: Limiting selectivity and capacity
at 313.15 K of phosphonium-based fluorinated
ionic liquids for the hexane
(1)/benzene (2) system, representing aliphatics/aromatics
separation
problems. References: [3C6C14P] [BF4] [19]; [3C6C14P]
[Tf2N] [19]; [3C6C14P] [(C2F5)3PF3] [20];
[3C6C14P] [PF6]
[19].
1.3. Ammonium-based Fluorinated ionic liquids
Limiting Selectivity
and Capacity
15
10
5
0
Ionic liquids with a common anion
[3C1C4N][Tf2N] [C13C8N][Tf2N]
Limiting Selectivity Limiting Capacity
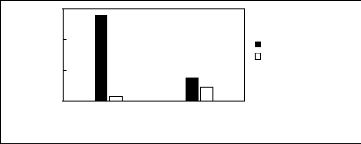
Figure H-4: Limiting selectivity and capacity
at 313.15 K of ammonium-based fluorinated
ionic liquids for the hexane
(1)/benzene (2) system, representing aliphatics/aromatics
separation
problems. References: [3C1C4N] [Tf2N] [21]; [C13C8N]
[Tf2N] [27].
Appendix H: Effect of structure on limiting selectivity and
capacity
2. Methanol/benzene separation problem
2.1. Imidazolium-based fluorinated ionic
liquids
|
Selectivity at infinite
dilution
|
2 1.6 1.2 0.8 0.4
0
|
|
[EMIM] + [BMIM] + ? [HMIM] + ? [MOIM] + *[C16MIM] +
|
[BF4]- [PF6] - [TfO] [SbF6] - [TFA] - [Tf2N]-
Anions
Figure H-5: Limiting selectivity at 313.15 K of
imidazolium-based fluorinated ionic liquids for the methanol (1)/benzene (2)
system, representing alcohols/aromatics separation problems.
References: [EMIM][BF4][2];[EMIM] [Tf2N]
[4]; [BMIM][BF4][6][7]; [BMIM] [Tf2N] [8]
;
[BMIM][TfO] [9] ; [HMIM][BF4] [12] ; [HMIM][PF6] [13] ; [HMIM] [Tf2N]
[16]; [MOIM][BF4] [17]
; [MOIM] [Tf2N] [16] ; [C16MIM][BF4] [18];
[EMIM][TfO] [28]; [MOIM][PF6] [29];
[BMIM][SbF6][30] ;[EMIM][TFA] [32].
2.2. Phosphonium-based fluorinated ionic
liquids
Limiting Selectivity
and Capacity
4
2
6
0
[3C6C14P][BF4] [3C6C14P][PF6] [3C6C14P][Tf2N]
[3C6C14P][(C2F5)2PF3]
Ionic liquids with a common cation
Limiting Selectivity Limiting Capacity
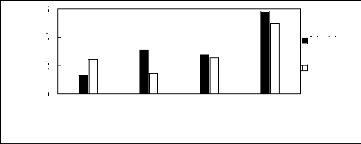
Figure H-6: Limiting selectivity and capacity
at 313.15 K of phosphonium-based fluorinated
ionic liquids for methanol
(1)/benzene (2) system, representing alcohols/aromatics separation
problems.
References: 3C6C14P] [BF4] [19]; [3C6C14P] [Tf2N] [19]; [3C6C14P]
[(C2F5)3PF3] [20];
[3C6C14P] [PF6] [19].
2.3. Ammonium-based fluorinated ionic liquids
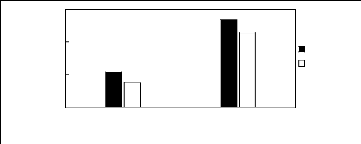
Limiting Selectivity
and Capacity
3
2
0
1
[3C1C4N][Tf2N] [C13C8N][Tf2N]
Ionic liquids with a common anion
Selectivity Capacity
Figure H-7: Limiting selectivity and capacity
at 313.15 K of ammonium-based fluorinated
ionic liquids for methanol
(1)/benzene (2) system, representing alcohols/aromatics separation
problems.
References: [3C1C4N] [Tf2N] [21]; [C13C8N] [Tf2N]
[27].
3. Methanol/acetone separation problem
3.1. Imidazolium-based fluorinated ionic
liquids
|
5 4 3 2 1 0
|
|
|
|
Limiting Selectivity
|
|
[EMIM] + ? [BMIM] + ? [HMIM] + [MOIM] + * [C16MIM] +
|
|
[BF4]- [PF6] - [TfO] - [SbF6] - [TFA] - [Tf2N]-
|
|
Anions
Figure H-8: Limiting selectivity at 313.15 K
of imidazolium-based fluorinated ionic liquids for
methanol (1)/acetone (2)
system, representing alcohols/ketones separation problems.
References:
[EMIM][BF4][2];[EMIM] [Tf2N] [4]; [BMIM][BF4] [7]
[2] ; [BMIM] [Tf2N] [8] ;
[BMIM][TfO] [9] ;
[HMIM][BF4][2] ; [HMIM] [Tf2N] [16];
[MOIM][BF4][17] ; [C16MIM][BF4] [18]
; [BMIM][SbF6]
[30] ; [BMIM][PF6] [31] .
Appendix H: Effect of structure on limiting selectivity and
capacity
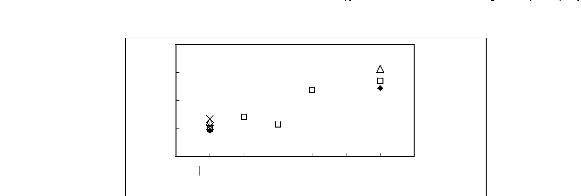
4
Limiting Capacity
3
2
1
0
[EMIM] + ? [BMIM] + ? [HMIM] + X [MOIM] + * [C16MIM] +
[BF4]- [PF6] - [TfO] - [SbF6] - [TFA] -
[Tf2N]-
Anions
Figure H-9: Limiting capacity at 313.15 K of
imidazolium-based fluorinated ionic liquids for
methanol (1)/acetone (2)
system, representing alcohols/ketones separation problems.
References:
[EMIM][BF4][2];[EMIM] [Tf2N] [4];
[BMIM][BF4][2] ; [BMIM] [Tf2N] [8] ;
[BMIM][TfO]
[9] ; [HMIM][BF4][2] ; [HMIM] [Tf2N] [16];
[MOIM][BF4][17] ; [C16MIM][BF4] [18]
; [BMIM][SbF6]
[30] ; [BMIM][PF6] [31].
3.2. Phosphonium-based fluorinated ionic
liquids
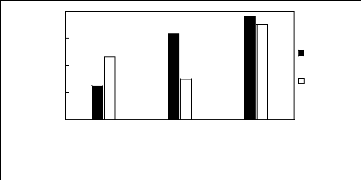
Limiting Selectivity and
Capacity
4
3
2
0
1
[3C6C14P][BF4] [3C6C14P][PF6] [3C6C14P][Tf2N]
]
Ionic liquids with a common cation
Limiting Selectivity
Limiting
Capacity
Figure H-10: Limiting selectivity and capacity
at 313.15 K of phosphonium-based fluorinated
ionic liquids for methanol
(1)/acetone (2) system, representing alcohols/ketones separation
problems.
References: 3C6C14P] [BF4] [19]; [3C6C14P] [Tf2N] [19]; [3C6C14P]
[(C2F5)3PF3] [20];
[3C6C14P] [PF6] [19].
3.3. Ammonium-based fluorinated ionic liquids
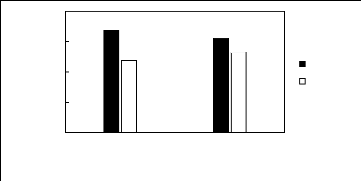
Limiting Selectivity and
Capacity
4
3
2
0
1
Ionic liquids with a common anion
[3C1C4N][Tf2N] [C13C8N][Tf2N]
Selectivity Capacity
Figure H-11: Limiting selectivity and capacity
at 313.15 K of ammonium-based fluorinated
ionic liquids for methanol
(1)/acetone (2) system, representing alcohols/ketones separation
problems.
References: [3C1C4N] [Tf2N] [21]; [C13C8N] [Tf2N]
[27].
4. n-hexane/hex-1-ene separation problem
4.1. Imidazolium-based fluorinated ionic
liquids
Anions
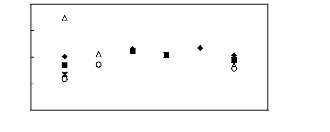
[EMIM] + [BMIM] + ? [HMIM] + ? [MOIM] + * [C16MIM] +
4
3
2
1
0
Selectivity at infinite dilution
[BF4]- [PF6] - [TfO] - [SbF6] - [TFA] -
[Tf2N]-
Figure H-12: Limiting selectivity at 313.15 K
of imidazolium-based fluorinated ionic liquids
for the n-hexane
(1)/hex-1-ene (2) system, representing paraffins/olefins separation
problems.
References: [EMIM][BF4][1][2];[EMIM] [Tf2N]
[3][5]; [BMIM][BF4] [6] [2] ; [BMIM] [Tf2N]
[4];
[BMIM][TfO] [9] ; [HMIMI[BF4] [12] ; [HMIM][PF6] [13] ; [HMIM]
[Tf2N] [14] ; [MOIM][BF4]
[17] ; [MOIM] [Tf2N] 16] ; [C16MIM][BF4]
[18] ; [EMIM][TfO] [28]; [MOIM][PF6] [29];
[BMIM][SbF6]
[30] ; [EMIM][TFA] [32].
Appendix H: Effect of structure on limiting selectivity and
capacity
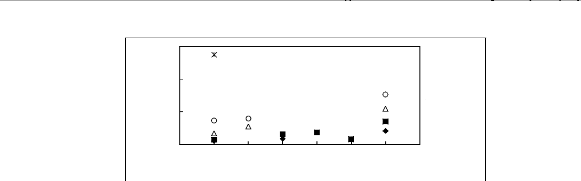
[BF4]- [PF6] - [TfO] - [SbF6] - [TFA] - [Tf2N]-
[EMIM] + [BMIM] + ? [HMIM] + ? [MOIM] + * [C16MIM]
0.6
Limiting Capacity
0.4
0.2
0
Figure H-13: Limiting capacity at 313.15 K of
imidazolium-based fluorinated ionic liquids for
the n-hexane (1)/hex-1-ene
(2) system, representing paraffins/olefins separation problems.
References:
[EMIM][BF4][2];[EMIM] [Tf2N] [5];
[BMIM][BF4][2] ; [BMIM] [Tf2N] [4] ;
[BMIM][TfO] [9] ;
[HMIM][BF4] [12] ; [HMIM][PF6] [13] ; [HMIM] [Tf2N] [14] ; [MOIM][BF4]
[17]
; [MOIM] [Tf2N] [16] ; [C16MIM][BF4] [18] ; [EMIM][TfO] [28];
[MOIM][PF6] [29];
[BMIM][SbF6] [30] ; [EMIM][TFA] [32].
4.2. Phosphonium-based fluorinated ionic
liquids
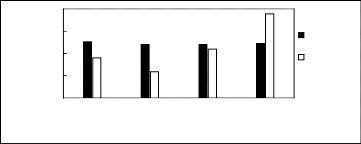
Limiting Selectivity
and Capacity
0.5
1.5
2
0
1
[3C6C14P][BF4] [3C6C14P][PF6] [3C6C14P][Tf2N]
[3C6C14P][(C2F5)2PF3]
Ionic liquids with a common cation
Limiting
Selectivity Limiting
Capacity
Figure H-14: Limiting selectivity and capacity
at 313.15 K of phosphonium-based fluorinated
ionic liquids for the n-hexane
(1)/hex-1-ene (2) system, representing paraffins/olefins
separation
problems. References: 3C6C14P] [BF4] [19]; [3C6C14P]
[Tf2N] [19]; [3C6C14P] [(C2F5)3PF3] [20];
[3C6C14P] [PF6]
[19].
4.3. Ammonium-based fluorinated ionic liquids
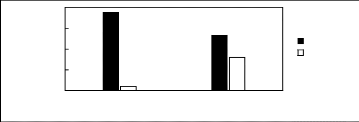
Limiting
Selectivity and
Capacity
0.5
1.5
2
0
1
[3C1C4N][Tf2N] [C13C8N][Tf2N]
Ionic liquids with a common anion
Selectivity Capacity
Figure H-15: Limiting selectivity and capacity
at 313.15 K of ammonium-based fluorinated
ionic liquids for the n-hexane
(1)/hex-1-ene (2) system, representing paraffins/olefins
separation
problems. References: [3C1C4N] [Tf2N] [21]; [C13C8N] [Tf2N]
[27].
4.4. Pyrrolidinium-based fluorinated ionic
liquids
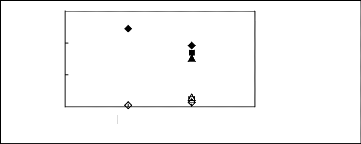
Limiting Selectivity
and Capacity
3
2
0
1
[TfO]- [Tf2N]-
Anions
S8 for [BMPyrr] +
n S8 for [HMPyrr] + ?Safer [MOPyrr] +
k8 for [BMPyrr]+
? k8 for [HMPyrr]+ ? k8 for
[MOPyrr]+
Figure H-16: Limiting selectivity and capacity
at 313.15 K of imidazolium-based fluorinated
ionic liquids for the n-hexane
(1)/hex-1-ene (2) system, representing paraffins/olefins
separation
problems. References: [BMPyrr] [TfO] [34]; [HMPyrr]
[Tf2N] [35]; [MOPyrr] [Tf2N] [35].
5. Benzene/butan-2-one separation problem
5.1 Imidazolium-based fluorinated ionic
liquids
|
2.5
2
1.5
1
0.5
0
|
|
|
|
Limiting Selectivity
|
|
[EMIM] +
? [BMIM] +
? [HMIM] + X [MOIM] +
* [C16MIM] +
|
|
[BF4]- [SbF6] - [Tf2N]-
|
|
Anions
Figure H-17: Limiting selectivity at 313.15 K
of imidazolium-based fluorinated ionic liquids
for the benzene
(1)/butan-2-one (2) system, representing ketones/aromatics separation
problems.
References: [EMIM][BF4][2];[EMIM] [Tf2N]
[4]; [BMIM][BF4] [2]; [HMIM][BF4][2] ;
[HMIM]
[Tf2N] [16] ; [MOIM][BF4] [17] ; [C16MIM][BF4] [18] ;
[BMIM][SbF6] [30].
|
2.5
2
1.5
1
0.5
0
|
|
|
|
Limiting Capacity
|
|
[EMIM] +
? [BMIM] + ? [HMIM] +
* [MOIM] +
? [C16MIM] +
|
|
[BF4]- [PF6] - [SbF6] - [Tf2N]-
|
|
Anions
Figure H-18: Limiting capacity at 313.15 K of
imidazolium-based fluorinated ionic liquids for
the benzene (1)/butan-2-one
(2) system, representing ketones/aromatics separation problems.
References:
[EMIM][BF4][2];[EMIM] [Tf2N] [4]; [BMIM][BF4]
[2]; [HMIM][BF4][2] ; [HMIM]
[Tf2N] [16] ; [MOIM][BF4] [17]
; [C16MIM][BF4] [18] ; [BMIM][SbF6] [30].
5.2 Phosphonium-based fluorinated ionic
liquids
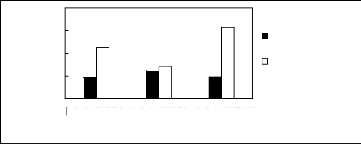
Limiting Selectivity
and
Capacity
4
3
2
0
1
[3C6C14P][BF4] [3C6C14P][PF6] [3C6C14P][Tf2N]
Ionic liquids with a common cation
Limiting Selectivity Limiting Capacity
Figure H-19: Limiting selectivity and capacity
at 313.15 K of phosphonium-based fluorinated
ionic liquids for the benzene
(1)/butan-2-one (2) system, representing ketones/aromatics
separation
problems. References: 3C6C14P] [BF4] [19]; [3C6C14P] [Tf2N] [19];
[3C6C14P]
[(C2F5)3PF3] [20]; [3C6C14P] [PF6] [19].
6. Ethanol/butan-2-one separation problem
6.1 Imidazolium-based fluorinated ionic
liquids
|
Limiting
Selectivity
|
4 3 2 1 0
|
|
[EMIM] +
n [BMIM] + ? [HMIM] + *[MOIM] +
? [C16MIM] +
|
|
[BF4]- [PF6] - [SbF6] - [Tf2N]-
Anions
|
|
Figure H-20: Limiting selectivity at 313.15 K
of imidazolium-based fluorinated ionic liquids
for the ethanol (1)/
butan-2-one (2) system, representing alcohols/ketones separation
problems.
References: [EMIM][BF4][2];[EMIM] [Tf2N]
[4]; [BMIM][BF4][7][2]; [HMIM][BF4][2] ;
[HMIM]
[Tf2N] [16] ; [MOIM][BF4] [17] ;
[C16MIM][BF4] [18] ; [BMIM][SbF6] [30] ; [BMIM][PF6] [31].
6.2 Phosphonium-based fluorinated ionic
liquids
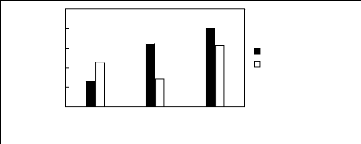
Limiting Selectivity
and Capacity
5
4
3
2
0
1
[3C6C14P][BF4] [3C6C14P][PF6] [3C6C14P][Tf2N]
Ionic liquids with a common cation
Limiting Selectivity Limiting Capacity
Figure H-21: Limiting selectivity and capacity
at 313.15 K of phosphonium-based fluorinated
ionic liquids for the ethanol
(1)/ butan-2-one (2) system, representing alcohols/ketones
separation
problems. References: 3C6C14P] [BF4] [19]; [3C6C14P] [Tf2N]
[19];
[3C6C14P] [(F5)3PF3] [20]; [3C6C14P] [PF6]
[19].
APPENDIX I: CORRELATION OF INFINITE
DILUTION
ACTIVITY COEFFICIENT, SELECTIVITY AND CAPACITY
1. Infinite dilution activity coefficient correlation
with the ionic liquid alkyl chain length 1.1. Imidazolium-based fluorinated
ionic liquids
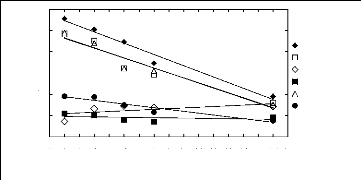
In (1113)
-1
4
2
5
3
0
1
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17
Nc
n-Hexane Hex-1-ene Ethanol Acetone Cyclohexane Benzene
Figure I-1: Variation of limiting activity
coefficients of various solutes depending on Nc, the
carbon number of the
alkyl chain attached to the methylimidazolium group with [BF4]-
anion.
Nc values corresponding to data points are: 2 for [EMIM]
[BF4][1][2], 4 for [BMIM] [BF4][2][6][7],
6 for [HMIM] [BF4][2][12], 8 for [MOIM]
[BF4][17] and 16 for [C16MIM] [BF4][18].
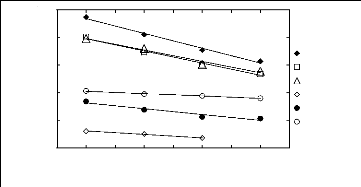
WS 13)
-0.5
-1.5
2.5
3.5
0.5
1.5
1 2 3 4 5 6 7 8 9
Nc
n-Hexane Hex-1-ene Cyclohexane Acetone Benzene Ethanol
Figure I-2: Variation of limiting activity
coefficients of various solutes depending on Nc, the
carbon number of the
alkyl chain attached to the methylimidazolium group with [Tf2N]-
anion.
Nc values corresponding to data points are: 2 for [EMIM] [Tf2N]
[3][4][5], 4 for [BMIM] [Tf2N]
[4][8], 6 for [HMIM] [Tf2N]
[14][15][16] and 8 for [MOIM] [Tf2N] [16].
1 2 3 4 5 6 7
Nc
n-Hexane Benzene Cyclohexane
5
4
3
In(11113)
2
1
0
Figure I-3: Variation of limiting activity
coefficients of various solutes depending on Nc, the
carbon number of the
alkyl chain attached to the methylimidazolium group with [TfO]-
anion.
Nc values corresponding to data points are: 2 for [EMIM] [TfO]
[28], 4 for [BMIM] [TfO] [9] and
6 for [HMIM] [TfO]
[33].
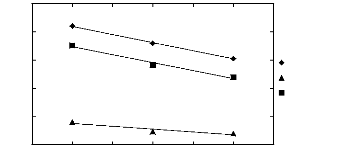
1.2. Pyrrolidinium-based fluorinated ionic
liquids
|
3
2
1
|
|
|
|
|
lii(LPi3 )
|
|
|
Hex-1-ene n-Hexane
|
|
|
|
|
3 4 5 6 7 8 9
Nc
Figure I-4: Variation of limiting activity
coefficients of n-hexane and hex-1-ene depending on
Nc, the carbon number of
the alkyl chain attached to the methylpyrrolidinium group
with
[Tf2N]- anion. Nc values corresponding to data points are: 4
for [BMPyrr] [Tf2N] [16],
6 for [HMPyrr] [35] and 8
for [OMPyrr] [Tf2N] [35].
2. Infinite dilution selectivity coefficient correlation
with the ionic liquid alkyl chain length 2.1. n-hexane/benzene
system
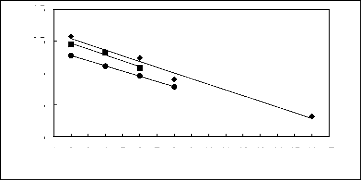
4.5
3.5
in (51:12)
2.5
1.5
0.5
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17
Nc
FILs containing [BF4]- FILs containing
[TfO]- ? FILs containing [Tf2N]-
Figure I-5: Variation of limiting
selectivities of n-hexane to benzene depending on Nc, the
carbon number of
the alkyl chain attached to the methylimidazolium group with common
[BF4]-
, [Tf2N]- and [TfO]- anions. Nc
values corresponding to data points are: 2 for [EMIM]
[BF4][1][2],
[EMIM] [TfO][28] and for [EMIM] [Tf2N]
[3][4]; 4 for [BMIM] [BF4][6][2], [BMIM]
[TfO][9] and
[BMIM] [Tf2N] [4][8]; 6 for [HMIM]
[BF4][12][2], [HMIM] [TfO][33] and [HMIM] [Tf2N]
[14][16];8
for [MOIM] [BF4][17] and [MOIM] [Tf2N] [16]
and 16 for [C16MIM] [BF4][18].
2-2. n-hexane/hex-1-ene system
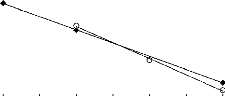
FILs containing [MIM] O FILs containing [MPyrr]

1 2 3 4 5 6 7 8 9
Nc
|
In (S°12)
|
0.8 0.7 0.6 0.5 0.4
|
Figure I-6: Variation of limiting selectivity
of n-hexane to hex-1-ene depending on Nc, the
carbon number of the alkyl
chain attached to the methylpyrrolidinium or methylimidazolium
group with
common [Tf2N]- anion. Nc values corresponding to data points are: 2
for [EMIM]
[Tf2N] [3][4]; 4 for [BMIM] [Tf2N] [4] and
[BMPyrr] [Tf2N] [16] ; 6 for [HMIM] [Tf2N] [14] and
[HMPyrr]
[Tf2N] [35] and 8 for [MOIM] [Tf2N] [16] and [OMPyrr]
[Tf2N] [35].
| 

