1.1.2 Chain flexibility
Higher chain stiffness results from a smaller number of
possible chain conformations; this can be caused by:
-greater stiffness of the main chain
-bigger side groups
-cross links
Some examples of the chain stiffness differences in the main
chain are:
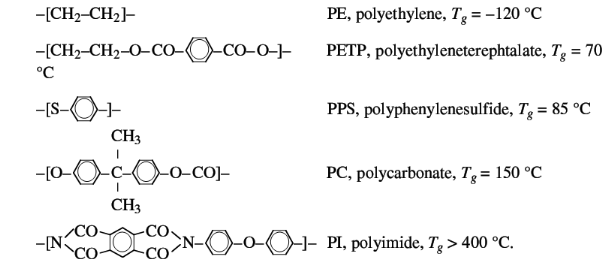
Some examples of the effect of side groups on the chain
flexibility are:
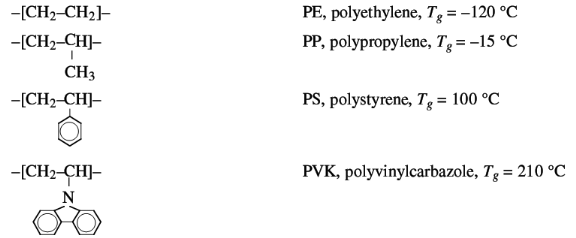
The increasing size of the side group effects a decrease of
chain flexibility and an increase of Tg.
1.1.3 Chain interactions
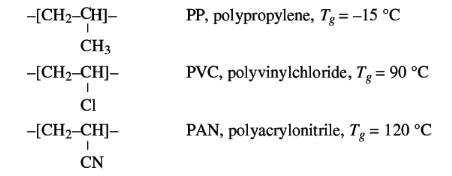
The strongest of molecular interaction are the dipole forces.
Their effect on Tg is illustrated by the series PP, PVC and PAN, in which the
chain mobility hardly varies because the side groups are of about equal size,
but in which, in the order of sequence mentioned, the dipole interaction
increase.
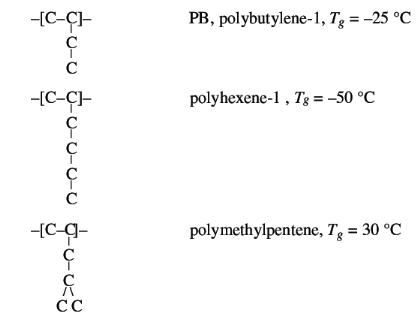
Interaction can be decreased by increasing the distance
between the chains, for instance with long side chains, which lower Tg. This
effect appears to be greater than the increase of chain stiffness, as shown in
the examples below:
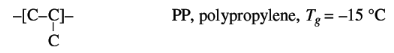
Approximate glass transition temperatures and melting point
of a few polymers are shown below:
Table1-1 glass transition temperatures of
common polymers
|
Polymer
|
LDPE
|
HDPE
|
PP
|
PVC
|
PS
|
PAN
|
PTFE
|
PMMA
|
PMMA
|
|
Tm(oC)
|
110
|
130
|
175
|
180
|
175
|
>200
|
330
|
180
|
30
|
|
Tg(oC)
|
-110
|
-110
|
-20
|
80
|
100
|
95
|
-110
|
105
|
-70
|
1.1.4 Intermolecular interaction
There are three types of intermolecular forces:
-Van Der Waals forces
-Dipole forces
-Hydrogen bond interactions
Although all such forces arise from the same fundamental
source i.e., interaction of negatively charged electrons and positively charged
nucleus yet they differ in magnitude, effective range and mode of operation.
Usually they are much smaller than the forces responsible for chemical
bonding.
1.1.5 Van Der Waals interactions
These interactions arise due to transfer polarization of
neutral molecules and are also known as London forces. Usually neutral
molecules have balanced number of negative electrons and positive charge on the
nucleus. Yet since electrons are in motion, the centre of density of negative
charge may not coincide with the centre of density of positive charge
continuously. A molecular thus acquires an electric dipole and can exert an
attraction for other similar molecules. Such interaction is known as van Der
Waals interaction.
A polarized molecule may induce the electric dipole in a
neutral molecule. However such polarized molecule continually reverts back to
neutral state and dipole is only transient. The greater the number of electrons
in a molecule and farther their distance from nucleus, the greater will be the
case of polarization and consently stronger Van Der Waals forces. These forces
vary inversely with the seventh power of the distance between molecules.
| 

