V.F. á 1/d7
Where V.F. is Van Der Waals forces and d is distance
separating the molecules. They are effective only over short intermolecular
distances.
1.1.6 Dipole-Dipole Interactions
Unequal sharing of electrons in covalent bonds results in
bonds dipoles and their magnitudes are indicated by the bond moments. As may be
expected the bond dipoles in different molecules attract each other resulting
in dipole-dipole interaction. These forces (D.F,) are governed by the
expression: D.F. á 1/d4
Where d is the distance between molecules, thus these forces
also are effectives only over short distances but have larger range than Van
Der Waals forces.
1.1.7 Hydrogen Bonds
It has been observed that when a hydrogen atom in a compound
is bonded to a highly electronegative atoms such as N, O, F, then marked
differences are observed in its usual properties like boiling point, solubility
etc. For example the boiling point of organic compounds usually increases with
increase in molecular weight but, though ethyl alcohol
C2H5OH (b.p.78.2o) and dimethyl ether
CH3-O-CH3 (b.p. -24.9 o) have the same
molecular weight, yet there is large difference in their boiling points.
The chemical properties of these compounds also differ as
compared to similar compounds not having hydrogen attached to N, O, F.
It is argued that when hydrogen is attached to such
electronegative atoms the bonding electrons are drawn strongly towards the
electronegative atom creating a dipole in the molecule. The hydrogen atom
therefore, acquires a small positive charge and becomes extraordinarily capable
of attracting a negatively charged atom of a molecule. This attraction results
in association of such molecules though the H-atom known as Hydrogen
Bond. This is represented by a dotted line. It has much less strength
than covalent bond and is essentially the result of electrostatic interactions,
delocalization effects and dispersion effects.
Hydrogen bonds are attractive interactions between a
positively charged hydrogen atom bonded to an electronegative element (the
donor: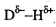 ), and a negatively charged atom with a lone pair of electrons (the
acceptor: ), and a negatively charged atom with a lone pair of electrons (the
acceptor: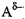 ) )
Table1-2 Functional group that can form
hydrogen bonds, arranged by element
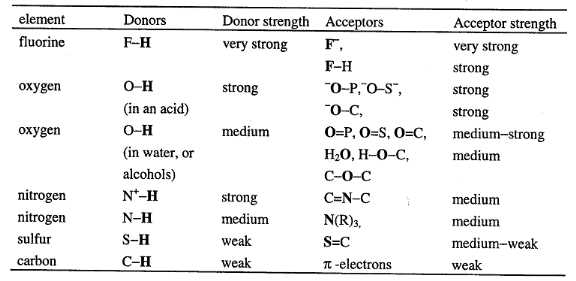
The strongest hydrogen bonding is formed between a strong
donor (like F-H and O-H in acid) and a strong acceptor.
The type of H-bonding resulting in association of two or more
molecules of the same or different compound is known as Intermolecular hydrogen
bonding. Intermolecular association trough hydrogen bond results in unusually
high boiling points of the liquids. Thus, the high boiling points of water,
alcohols, amines and acids as compared to monomeric molecules of comparable
molecular weight may be explained on the basis of H-bonding.
Intermolecular Hydrogen bonding is the formation of H-bonging
within the molecule itself. Ethylacetoacetate, salicylaldehyde and
o-nitrophenol are example of this type.
1.2 Styrene and Maleimide
copolymer
Polystyrene is one of a common polymer. It is
very easy to produce and proceed, so very cheap, and has majority of properties
for usage in common life. However, its glass transition temperature (Tg) is
only 100 degree Celsius, which leads to limit its applications.
Polymers with high glass transition temperature are attractive
for industrial polymer science because of their strong economic rewards that
may arise from their potential application [1]. As mentioned above,
two factors governs Tg of polymers, chain flexibility and chain interaction.
Copolymerization is a best way to change both of them. In the case of
copolymers, the final value of a given property; e.g. the melting point or the
glass transition temperature does depend on both of monomer structures and the
composition of them, also the others[2]. The existing methods used
to improve Tg of Polystyrene are the copolymerization and control of its
configuration. Incorporation of a few of another stiff monomer shows less
improvement in Tg of polystyrene, because the Tg is a function of content of
stiff monomer. More content of stiff monomer makes Tg of styrene copolymer
higher, meanwhile many good properties, for instance, stiffness, transparent,
and processing property, will be lost. Isotactic polystyrene has very high
Tg(above 220oC). However, it is difficult to process and,
furthermore, it is gotten by much more complicated coordination polymerization
route, not by the easy free radical polymerization.
Styrene molecule or derivates are chemically modified by free
radical polymerization to obtain new products with various potential
applications and properties [3]. Copolymerization of maleimides with
styrene provides the possibility of synthesizing higher and thermally stable
polymers. In addition to that the processability of maleimide polymer can also
be enhanced by the incorporation of more flexible units within the polymer
backbone [1].
Styrene-Maleimide copolymer (SMA) have been found to have
versatile applications in many industries ranging from aerospace to the
microelectronics field [2]. During the past several years many
reports and researches in the free radical copolymerization of styrene with
maleimide have emerged[2-5].
| 


