1.4 2,6-diaminopyridine molecule
2,6-diaminopyridine is analogous melamine. It can also form
the triple hydrogen bond with SMA.
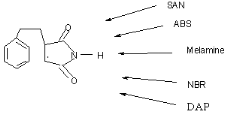
Fig 1-5 Potential precursors of the triple
hydrogen bonded Styrene Maleimide couple
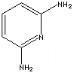
Fig 1-6 2,6-Diaminopyridine
PYRIDINE is a heterocyclic aromatic tertiary amine characterized
by a six-membered ring structure composed of five carbon atoms and nitrogen
which replace one carbon-hydrogen unit in the benzene ring
(C5H5N). The simplest member of the pyridine family is
pyridine itself. It is colorless, flammable, toxic liquid with a unpleasant
odor, miscible with water and with most organic solvents, boils at 115
oC. Its aqueous solution is slightly alkaline. Its conjugate acid is
called pyridinium cation, C5H5NH+, used as a
oxidation agent for organic synthesis. Pyridine is a base with chemical
properties similar to tertiary amines. Nitrogen in the ring system has an
equatorial lone pair of electrons that does not participate in the aromatic
pi-bond. Its aqueous solution is slightly alkaline. It is incompatible and
reactive with strong oxidizers and strong acids, and reacts violently with
chlorosulfonic acid, maleic anhydride, oleum, perchromates, b-propiolactone,
formamide, chromium trioxide, and sulfuric acid. Liquid pyridine easily
evaporates into the air. If it is released to the air, it may take several
months to years until it breaks down into other compounds. Usually, pyridine is
derived from coal tar or synthesized from other chemicals, mainly acetaldehyde
and ammonia.
Pyridine and its derivatives are very important in industrial
field as well as in bio chemistry. 2,6-Pyridinediamine is used as an
intermediate for the synthesis of analgesic drugs. Phenazopyridine is an
example derived from 2,6-Pyridinediamine.
1.5 The thesis work
The use of secondary interactions a well-accepted strategy to
enhance the miscibility of immiscible polymers, and miscible polymer mixtures
based on hydrogen bonding. This is result in interesting and enhanced polymeric
properties[12,13]. The use of hydrogen bonds offers the advantage
that they involve distinct donor and acceptor sites, and are very directional.
In that way they offer more possibilities for structural design than forces
that are symmetric and non-directional as e.g. ion-ion interactions.
In our case, we will improve Tg of polystyrene based on
secondary interactions. Knowledgably crosslinking can increase Tg of polymers.
However, nobody tried to use the method because few crosslinkage in polystyrene
will lead to worse rheological property.
The arrangement of hydrogen bond donors and acceptors of
maleimide, which are involved in hydrogen bonding in the complex with melamine,
suggest that it should be possible to complex one melamine molecule to three
imides units.
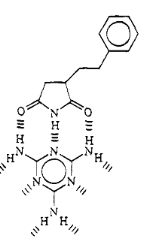
Fig 1-7 Proposed Structure of
Maleimide/styrene interactions with melamine
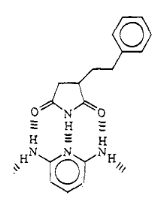
Fig 1-8 Proposed Structure of
Maleimide/styrene interactions with 2,6 diaminopyridine
In general, the binding strength of multiple hydrogen bonded
complexes is depending on the strength of the individual hydrogen bonds in the
array, and the number of hydrogen bond. Different hydrogen Donor(D) and
hydrogen acceptor (A) arrays can be obtained e.g. a triple bond( DDD-AAA array,
DDA-AAD array, DAD-ADA array)[12]. The DAD-ADA triple hydrogen bond array is
frequently used in organic chemistry due to its synthetic availability.
The use of a hydrogen bonding unit possessing two or more
interaction sites should result in network formation. It is a new, simple,
economical method to prepare polystyrene with higher Tg based on hydrogen
bonding crosslinkage between melamine and imide in the polystyrene prepared by
free radical copolymerization of styrene and few amount of maleimide. The
thermoreversible properties of hydrogen bond make the polystyrene have good
rheological properties with higher Tg.
In order to reveal the interaction between imide and melamine,
we used 2,6-diaminopyridine to replace melamine to complex with imide from SMA.
Both of them should complex with imide by DAD-ADA arrays shown in the Fig.1-7
and Fig.1-8.
| 


