3.3.2 Chromatographic separation method
3.3.2.1 General description of chromatography
Chromatography is a separation,
identification, purification and quantification technique that dates from the
work of the Russian chemist Mikhail Tswett in 1903. There are a variety of
chromatography techniques, in common use, all of which work on a similar
principle. The mixture to be separated is dissolved in a solvent, called the
mobile phase, and passed over an adsorption material, called the stationary
phase, which is fixed in place in a column or on a solid surface. Those
components that are strongly retained by the stationary phase move only slowly
with the flow of mobile phase. In contrast, components that are weakly held by
the stationary phase travel rapidly. As a consequence of these differences in
mobility, sample components separate into discrete bands, or zones, that can be
analyzed qualitatively and/or quantitatively. From the chromatogram, several
parameters like the retention time can be deduced to characterize the
separation and the efficiency (Niessen, 1999).
Chromatographic processes can be classified according to the
type of equilibration process involved, which is governed by the type of
stationary phase. Various bases of equilibration are: adsorption
(TLC), partition (HPLC), ion
exchange (IEC), Molecular Exclusion
Chromatography and affinity chromatography.
High-performance liquid chromatography is the most widely
used of all the analytical separation techniques. The reasons for the
popularity of the method is its sensitivity, its ready adaptability to accurate
quantitative determinations, its suitability for separating volatile species,
similar polarties components or thermally fragile ones, and above all, its
widespread applicability to substances that are of prime interest to industry,
to many fields of science, and to the public.
Discussions about HPLC methods often revolve around the
internal diameter (id) or bore of the column to be used. Standard bore columns
have an id of 4 or 5 mm while narrow bore are half that or less. Packed with
the same materials, the narrow bore column will require less solvent for the
same resolving power since the analytes can be eluted at a lower flow rate,
under 0.5 ml/min, than the 2 to 3 ml/min used for standard bore. Narrow bore
columns are 4 to 6 times more sensitive (b) using the injection volume required
for a standard bore column (a). The eluting analytes can be detected by a
variety of techniques, the most universal being UV-visible absorbance (1)
which, with diode-array (DAD) technology provides spectral confirmation in the
third dimension. Particular analytes have specific physical characteristics
that enable detection based on fluorescence, phosphorescence or
chemiluminescence (2), refractive index or electrochemical HPLC can be coupled
with others analytical methods like HPLC-mass spectrometry (HPLC-MS), HPLC-nuclear magnetic resonance (HPLC-NMR).
3.3.2.2 Introduction to HPLC/MS technique
In its simplest form, MS (mass spectrometry), a technique
used to characterize and separate ions by virtue of their mass/charge
(m/z) ratios can be helpful in structure determination as the
fragmentation can give useful informations about the structure. Mass
spectrometry data from HLPC-MS has two dimensions: Time and Mass. Time
describes the isolated time of molecule (retention time, Rt) and Mass
represents the mass/charge ratios. Mass spectrometry, especially
HPLC/MS/MS, is an important and quite useful technique for the detection,
identification, quantitation and analysis of small pharmaceutical molecules,
peptides, proteins, and oligonucleotides and their metabolites and degradants.
There are several common modes of obtaining mass spectra. These include:
Time-of-flight (TOF), quadrupole, ion trap, magnetic sector, and combinations
of these. Ionization techniques commonly used in biotechnology and
pharmaceutical analysis for non-volatile samples include Matrix-Assisted Laser
Desorption/Ionization (MALDI), Electrospray Ionization (ESI), Inductively
Couple Plasma (ICP), electron capture ionization (ECI), Atmospheric Pressure
Chemical Ionization (APCI) and Fast Atom Bombardment (FAB). Each technique has
its own set of advantages and disadvantages. That is, no one technique will
solve all problems.
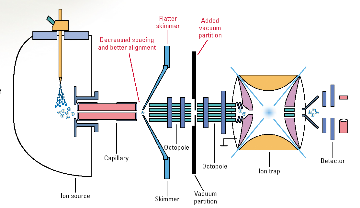
An Agilent1100 Series LC/MSD system consists of an ion trap
mass spectrometer and a HPLC as shown in Figure 4-. The mass spectrometer is
equipped with electrospray (ESI) and atmospheric pressure chemical ionization
(APCI) ion sources and is able to operate in positive and negative ion modes.
Samples can be analyzed by direct injection into the ion source or following
separation using high performance liquid chromatography. Both qualitative and
quantitative analyses are available by using full scan, single ion or selected
reaction monitoring. A variety of tandem mass spectrometry experiments can be
performed with ions produced by ESI and APCI methods (Figure 4-1).
Figure 3-1 Schematic diagramm of Agilent 1100
Series LC/MSD Trap (Agilent Technologies, 2001).
|

