Chapter 4 Results and discussion
4. 1 Choice of the medium
One of the reasons contributed to the conflicting
results reported in the literature about interaction hemin-antimalarial drug
interactions is the inappropriate choice of the working medium. It has been
well shown that the study of Fe(III)PPPPIX in aqueous solution is problematic
because of its tendency to aggregate or dimerize. As can be seen from Figure 7,
the spectra (b) of hemin in alkaline aqueous solution showed a large band from
350 to 400 nm which is attributed to an oxodimere represented as
(H2O)Fe-O-Fe(H2O), whereas hemin was monomeric and exhibited a sharp Soret peak
with a maximum at 396-398 nm (50 % PREG or 50 % EG), at 400 nm (25 % DMSO, 80
% ethanol), at 402 nm (40 % DMSO), at 404 nm (DMSO). The slight shift observed
towards longer wavelength is due to the change of medium.
Practically, propylene glycol mixture presents the same
thermodynamic advantage as ethylene glycol mixture and is much less toxic than
the latter. It was used to study both interaction pairs of hemin-quinoline and
hemin-artesunate. Since Artemisinin and dihydroartemisinin are insoluble in
both propylene glycol and 25 % DMSO, the bonding of hemin with artemisinin
compounds was investigated in 40 % DMSO aqueous solutions. Because of their
density, polarity, wide temperature range of the liquid state and ability to
have bonding hydrogen with water molecules, DMSO and PREG mix easily with
water. Particularly, DMSO is an extraordinarily efficient solvent for many
kinds of substances including both organic and inorganic compounds. The heat of
mixing of DMSO and water indicates there are stronger interactions between DMSO
and water than between DMSO molecules (Yu and Quinn, 1994). At high DMSO
concentrations, water-structure is disrupted due to the formation of the
DMSO-water complexes.
Figure 4-1 Spectra of hemin solutions in
different mediums at 25oC.

The spectrum range from 300 to 500 nm and 250 to
650 nm were selected to study the interactions of hemin-quinoline and
hemin-artemisinin, respectively. This is because the induced spectral
modifications in the presence of the drugs are more significant in this range
than in the remainder of the UV-visible region.
4. 2 Choice of buffers
Tris-HCl buffer was preferred to phosphate buffer because the
latter showed some incompatibilty in terms of solubility (formation of
precipitate) in 40 % DMSO and was not suitable when pH>8, although it was
well used both in 25 % DMSO and water-propylene glycol mixture at pH 7.4.
4.3 Binding reaction of hemin with chloroquine, quinine and
quinidine in water-propylene glycol mixture.
More specifically, the wavelength of 396 nm was selected to
determine the constants of complexation because of the greatest variation of
the optical density observed in the presence of the antimalarial drugs.
Titration of hemin by increasing amount of drugs in mixed
water-propylene glycol solutions gives typical spectral changes as exemplified
in Figure 4-2. They are similar to those observed on deuterohemin-quinine,
hemin-chloroquine and hemin-quinine interactions in other mediums
(Constantinidis and Satterlee, 1988; Gushimana et al., 1993, 1996).
The absorption band centered around 332 nm is from the
quinoline derivative and that centered at 396 nm is from hemin. As can be seen
from Figure 4-2, addition of chloroquine drug modifies markedly the hemin
spectrum, but the peak maximums are still at about 396 nm. This indicates that
the complexation does not involve significant modifications on the structure of
the porphyrin ring of the ferriprotoporphyrin IX.
Another feature that can be seen for all the three drugs is
the appearance of an isosbetic point located at around 350 nm on the titration
curves. The experimental data were fitted into a 1:1 complex model as described
mathematically in Eq. (3-9). What are shown in Figure are selected such results
with the total drug concentration as the only changing parameter. It can be
seen that the extinction of the hemin solution decreases with increasing total
drug concentration.
This trend is consistent with previous results and can be
attributed to complex formation between the drug and hemin (Constantinidis and
Satterlee, 1988; Gushimana et al., 1993, 1996).
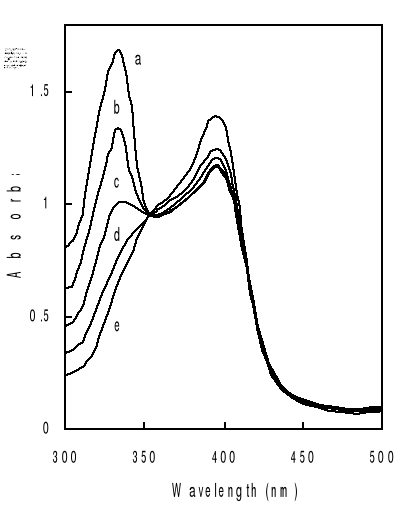
The solid curves in the figure are fitted data with the
experimental results according to Eq. (3-9). Correlation coefficients of the
nonlinear fittings are better than 0.9, which implies that the titration curves
can be well described by the 1:1 complexation scheme. Similar variation in
absorbance of hemin at 396 nm as function of total drug concentration has been
obtained at other values of pH and the results are also consistent with the
formation of 1:1 complex.

Values of binding constants at various pH obtained from these
titration curves are summarized in Table 4-1. As highlighted by values of
binding constants in Table 4-1, K values are in the same order of
magnitude as those obtained in water-ethylene glycol mixture (Gushimana et al.,
1993, 1996).
Table 4-1 Binding
constant of hemin-drug complexes at various pH.
|
K (105 M)
|
|
pH
|
Hemin-chloroquine
|
Hemin-quinine
|
Hemin-quinidine
|
|
9.0
|
0.170.03
|
0.050.01
|
2.170.43
|
|
8.1
|
0.220.04
|
0.150.03
|
4.170.83
|
|
7.4
|
0.330.06
|
0.110.03
|
1.770.94
|
|
6.8
|
0.400.10
|
0.110.02
|
2.870.92
|
In fact, the complexation of ferriprotoporphyrin IX with the
drug is believed to play the role to bring back the hemin into solution in
order to prevent it from polymerization. The ability of quinoline drug to
complex with hemin will inhibit the formation of hemozoin (-hematin) in vivo.
The drug that has a greater affinity with hemin should maintain more hemin in
solution and is thus more effective. This means that quinidine should have the
highest efficiency, then comes chloroquine, and finally quinine, based on the
data in Table 4-1. But in practical applications, an opposite trend is
observed, probably due to the emergence of new resistant strains of malaria
parasites against the existing and commonly used antimalarial drugs.
As a matter of fact, in some areas (the case in D.R.Congo, for
example) quinine appears more effective than chloroquine. This proves that the
strength of haematin-quinoline interactions does not directly correlate with
antiplasmodial activity. This indicates that haematin binding is a necessary,
but not sufficient requirement for antiplasmodial activity (Egan et al.,
1994).
Scheme 4-1 Structures of three
quinoline-based drugs, quinine (1), quinidine (2) and chloroquine (3).
In regard to the molecular basis of the hemin-drug
interactions, rather less is known about the structures of these complexes. In
fact, the complexes between 4-aminoquinolines and hemin are almost certainly
p-p complexes (Egan et al., 1994).
This means that there is an interaction between the aromatic
ring of the quinoline and the porphyrin structure. In addition, hydrophobic
interaction, electronic and steric factors also play important roles in
influencing the structures of such complexes. Results from the present study
show that chloroquine interacts more strongly with ferriprotoporphyrin IX than
quinine does, indicating some additional interaction of the side chain of the
quinoline with Fe(III)PPIX. This finding rejoins the result of Egan and
co-workers which reported the association constants of chloroquine (log
K= 5.52) and quinine (log K= 4.10) in 40% aqueous DMSO at pH
7.5 (Egan et al., 2000). It is suggested that the flexible side aliphatic chain
of the chloroquine structure, which is less crowded than that of the stiff
quiniclidine group of the quinine structure, stabilizes hemin-chloroquine
interaction. It is also supposed also that a hydrogen-bonding interaction
between the side-chain amine group of chloroquine and the heme propionate group
may play a role in the hemin-chloroquine complex stability. More likely, there
may be some direct Van der waals interaction between the side chain of
quinoline and the porphyrin ring. In addition, the stability of these complexes
is supported by computational results. A molecular mechanics study of the
interaction between chloroquine and an iron-porphyrin model for
N-acetylmicroperoxidase-8 revealed a minimum energy arrangement with
coplanar interaction of the quinoline and iron-porphyrin ring, but could not
define a preferred conformation for the complex (Marques, 1996).
It is interesting to note that the conformation of drugs
affects their affinity with hemin. As can be seen from Scheme 1, quinine
differs from quinidine only at positions C-8 and C-9, the former has 8S9R
structure and the latter has 8R9S structure (Ribeiro, 1997). The data showed
significantly different affinity to hemin of the two chiral isomers.
Further more, it can also be seen that K values are
pH-dependent. That dependence is probably due to acido-basic equilibrium
influence on electrostatic interactions between hemin and the drugs. Due to
their different pKa values, reacting partners have different electric
charge at different pH values (Constantinidis and Satterlee, 1988;
Gushimana et al., 1993; Kuhn, 1995).
| 
